Periodic Element B 56 Barium Ba Digital Art by Organic Synthesis Fine Art America
Barium Chemical 56 element of periodic table. Molecule And Communication Background. Chemical Ba
Element Barium (Ba), Group 2, Atomic Number 56, s-block, Mass 137.327. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main content .. This is a 'barium meal' or 'barium enema'. Barium is a heavy element and scatters X-rays, so as it passes through the body the stomach and intestines can.

barium,lybdum,o(第15页)_大山谷图库
Protons and Neutrons in Barium. Barium is a chemical element with atomic number 56 which means there are 56 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs.

Barium Ba (Element 56) of Periodic Table Elements FlashCards
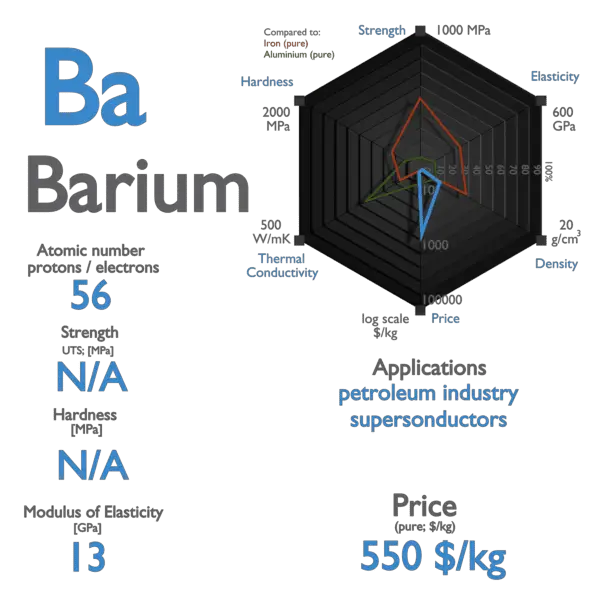
Barium is a soft metal having silvery grey metallic lustre with a pale yellow tint. The melting point of barium is 727 °C and its boiling point is 1845 °C. The atomic mass of barium is 137.33 u and its density is 3.51 g/cm 3, which is quite dense among all the other alkaline earth metals on the periodic table.

Periodic Element B 56 Barium Ba Digital Art by Organic Synthesis Fine Art America
Barium is a metallic element, soft, and when pure is silvery white; it belongs to the alkaline earth group, chemically resembling calcium. The metal oxidizes very easily and should be kept under petroleum or other suitable oxygen-free liquids to exclude air. It is decomposed by water or alcohol. Uses The metal is used as a "getter" in vacuum tubes.

Barium Ba, Chemical Element Sign. 3D Rendering Isolated on White Background Stock Illustration
Barium has a melting point of 725°C, a boiling point of 1640°C, and a specific gravity of 3.5 (20°C), with a valence of 2. Barium is a soft metallic element. In its pure form, it is silvery white. The metal oxidizes readily and should be stored under petroleum or other oxygen-free liquids. Barium decomposes in water or alcohol.

Element THE WORLD ALOHA
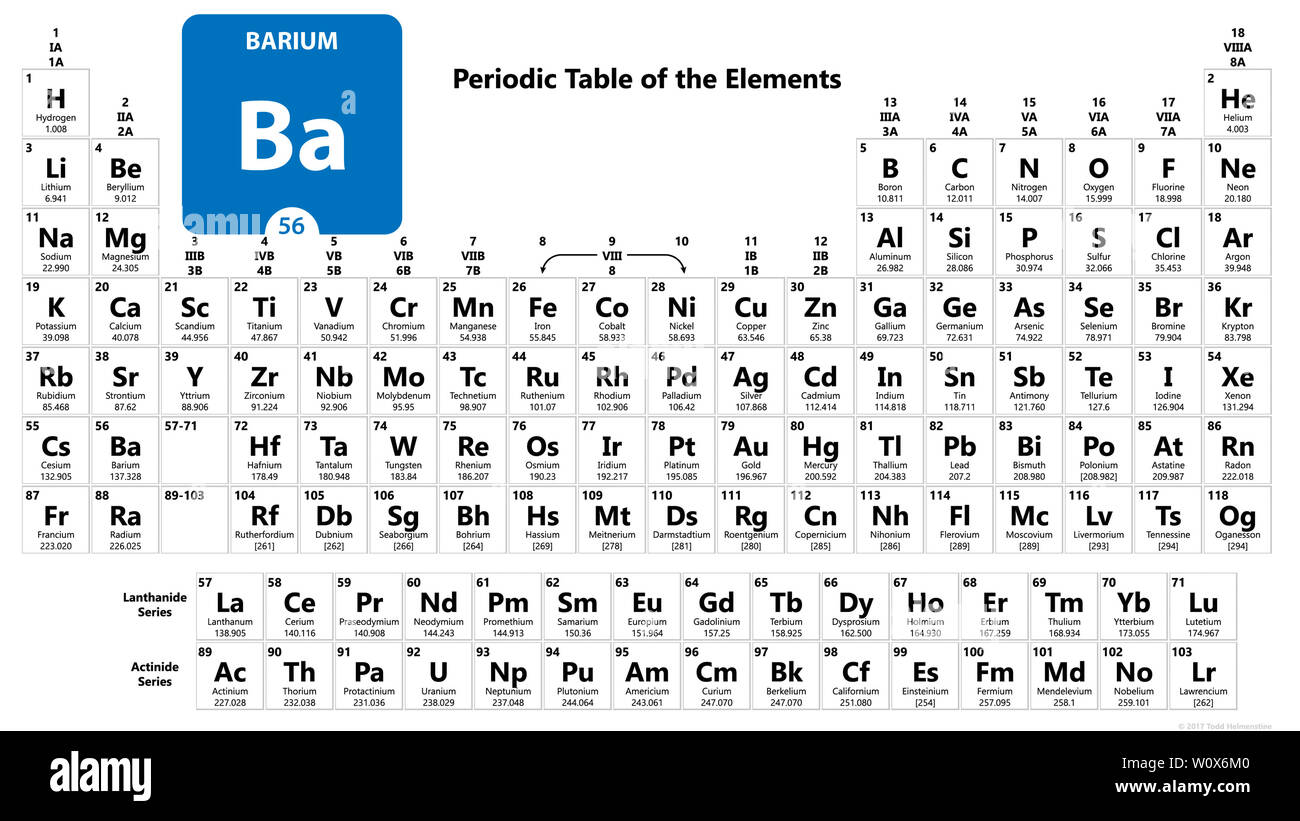
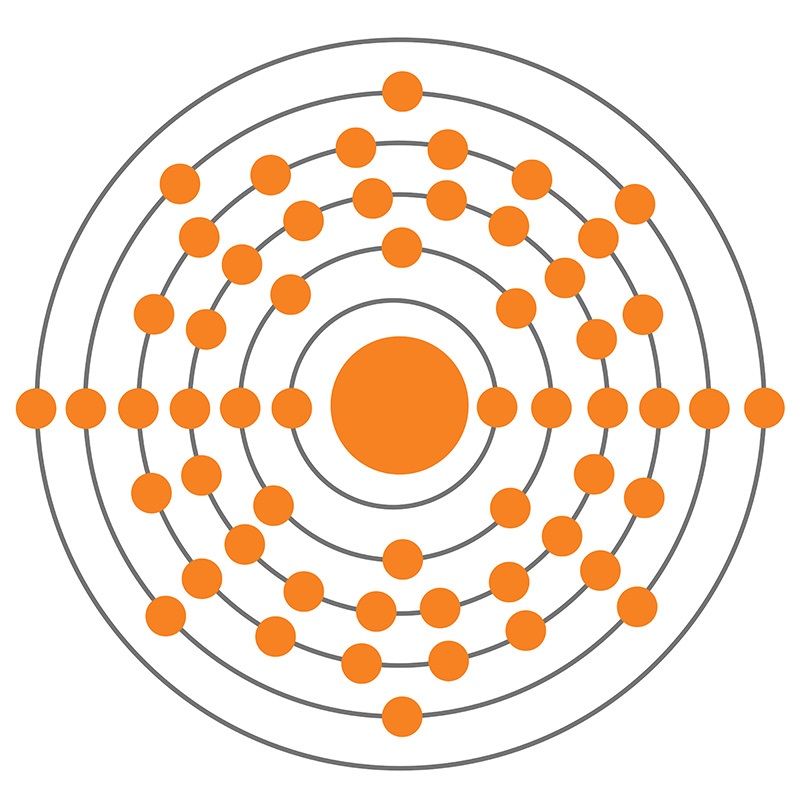
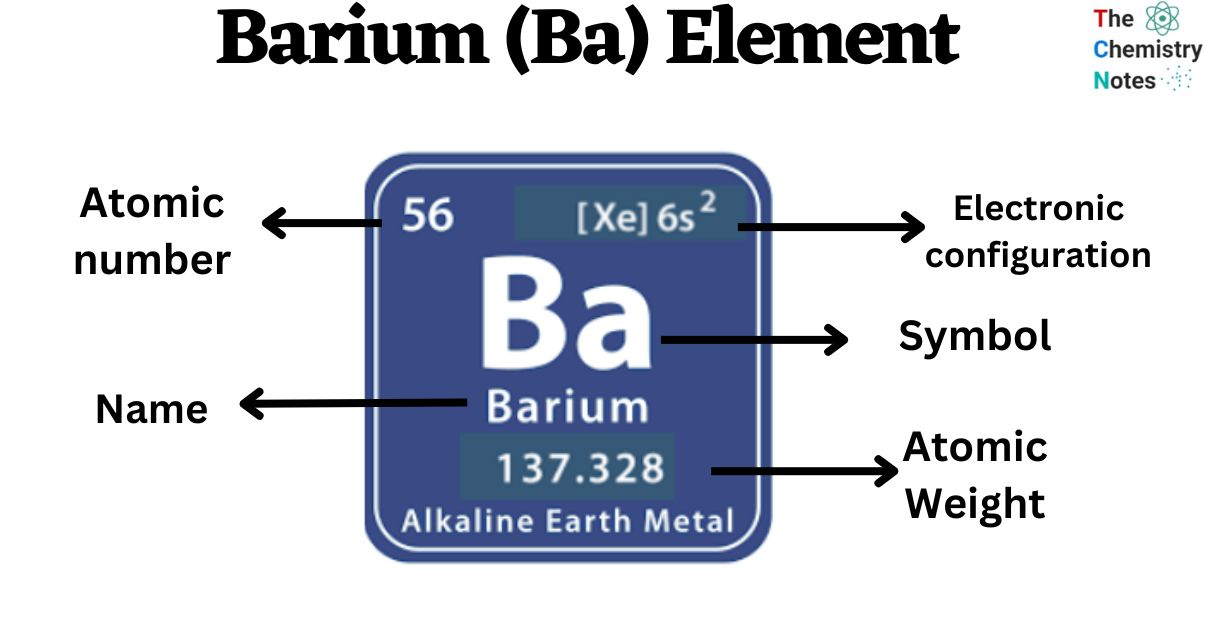
Barium is the 56th element in the periodic table and has a symbol of Ba and atomic number of 56. It has an atomic weight of 137.327 and a mass number of 138. Barium has fifty-six protons and eighty-two neutrons in its nucleus, and fifty-six electrons in six shells. It is located in group two, period six and block s of the periodic table.

Barium Element Barium TShirt TeePublic
Barium is the fifth chemical element in the periodic table of elements with the atomic number 56. Being a member of the alkaline earth metals family, this soft metal is divalent, electropositive, and reactive, and supports the formation of many compounds with other chemical elements, especially carbon, oxygen, and sulphur.

"Periodic Table element Barium Ba number 56" Poster for Sale by PeriodicBliss Redbubble
Element 56 of Periodic table is Barium with atomic number 56, atomic weight 137.327. Barium, symbol Ba, has a Body Centered Cubic structure and Silver color. Barium is a Alkaline Earth Metal element. It is part of group 2 (beryllium family).
Barium (Ba) AMERICAN ELEMENTS
Barium is a chemical element with symbol Ba and atomic number 56. Classified as a n alkaline earth metal, Barium is a solid at room temperature. 56 Ba Barium View All Properties H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga

Barium Symbol Element Number 56 Of The Periodic Table Of The Elements Chemistry Stock
Barium(atomic number 56, symbol Ba) is a chemical element and an alkaline earth metal with a soft silvery color. Because of its reactivity with air, this metallic metal is not observed in its pure form in nature. Barium is found in naturally occurring minerals. Among them are witherite, which is a barium carbonate, barite, and barium sulfate.
Barium (Ba) Element Properties, Uses, Reactions, Effects
barium , Chemical element, one of the alkaline earth metals, chemical symbol Ba, atomic number 56. It is very reactive and in compounds always has valence 2. In nature it is found chiefly as the minerals barite (barium sulfate) and witherite (barium carbonate).
Bario Taringa!
Download the Periodic Table. Barium (Ba) is a soft silvery white coloured metal that has the atomic number 56 in the periodic table. It is an Alkaline earth metal and is located in Group 2 of the periodic table. it has the symbol Ba.
What is Barium Properties of Barium Element Symbol Ba
The atomic number of barium is 56 and atomic weight of 137.327 and belong to Group 2 periodic table. Barium chemically resembles, magnesium calcium and strontium but is more reactive. Reaction with chalcogens are highly exothermic. It combines easily with oxygen, the halogens, and other non-metals also reacts with water and most acids.

"Periodic Table element Barium Ba number 56" Sticker for Sale by PeriodicBliss Redbubble
Barium is a chemical element with atomic number 56 which means there are 56 protons and 56 electrons in the atomic structure. The chemical symbol for Barium is Ba. Barium is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element.

Element Barium Coasters Cork, Puzzle & Tile Coasters CafePress
Naturally occurring barium (56 Ba) is a mix of six stable isotopes and one very long-lived radioactive primordial isotope, barium-130, identified as being unstable by geochemical means (from analysis of the presence of its daughter xenon-130 in rocks) in 2001. This nuclide decays by double electron capture (absorbing two electrons and emitting two neutrinos), with a half-life of (0.5-2.7)×.

Yeezy Quantum Barium Takes After a Chemical Element & 3 Other Yeezys!
Barium (Ba) [56] — Chemical Element — Periodic Table Barium Metal ⬇ Physical data Electronic data Shells: 2, 8, 18, 18, 8, 2 Orbitals: [Xe] 6s 2 Electronegativity: 0.9, 1.0 1. Ionization potential: 5.2117 eV 2. Ionization potential: 10.004 eV 3. Ionization potential: -- eV Oxidation states: 2 Electrical conductivity: 0.030 10^6 Thermal data