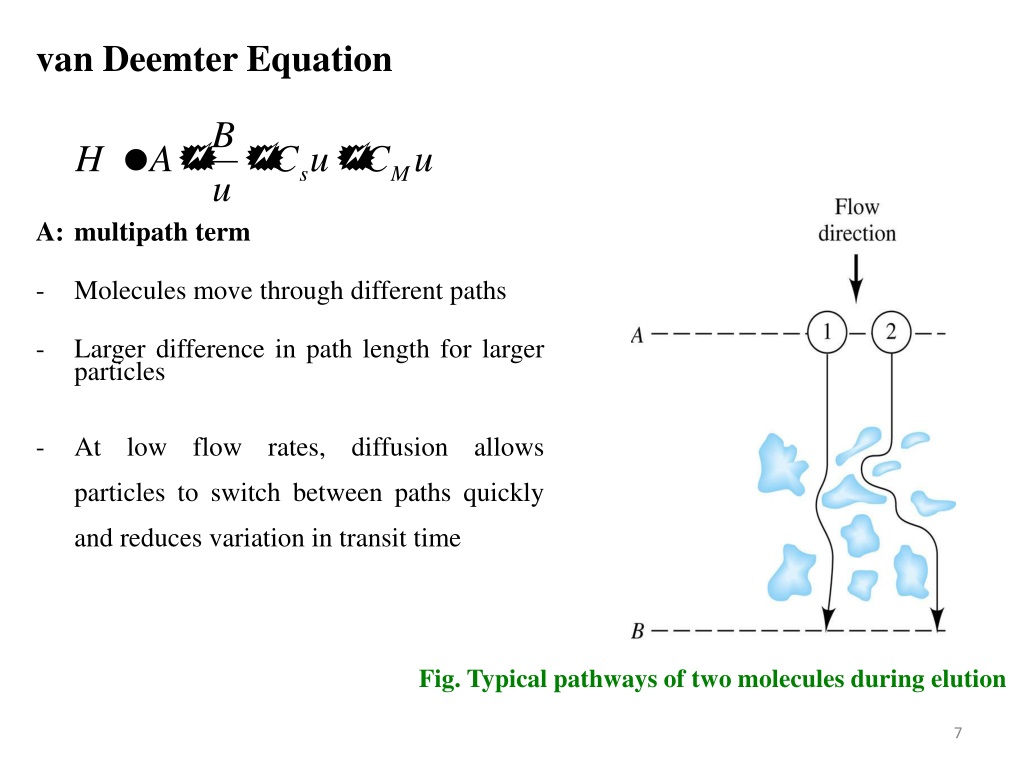
Van Deemter equation
Van Deemter equation The Lockdown guide
What we will develop as we analyze the four contributions to broadening above is an equation, which was first known as the van Deemter equation (J. J. van Deemter described the first treatment of this for chromatographic systems in 1956), that relates these four terms to the reduced plate height.
Van Deemter equation
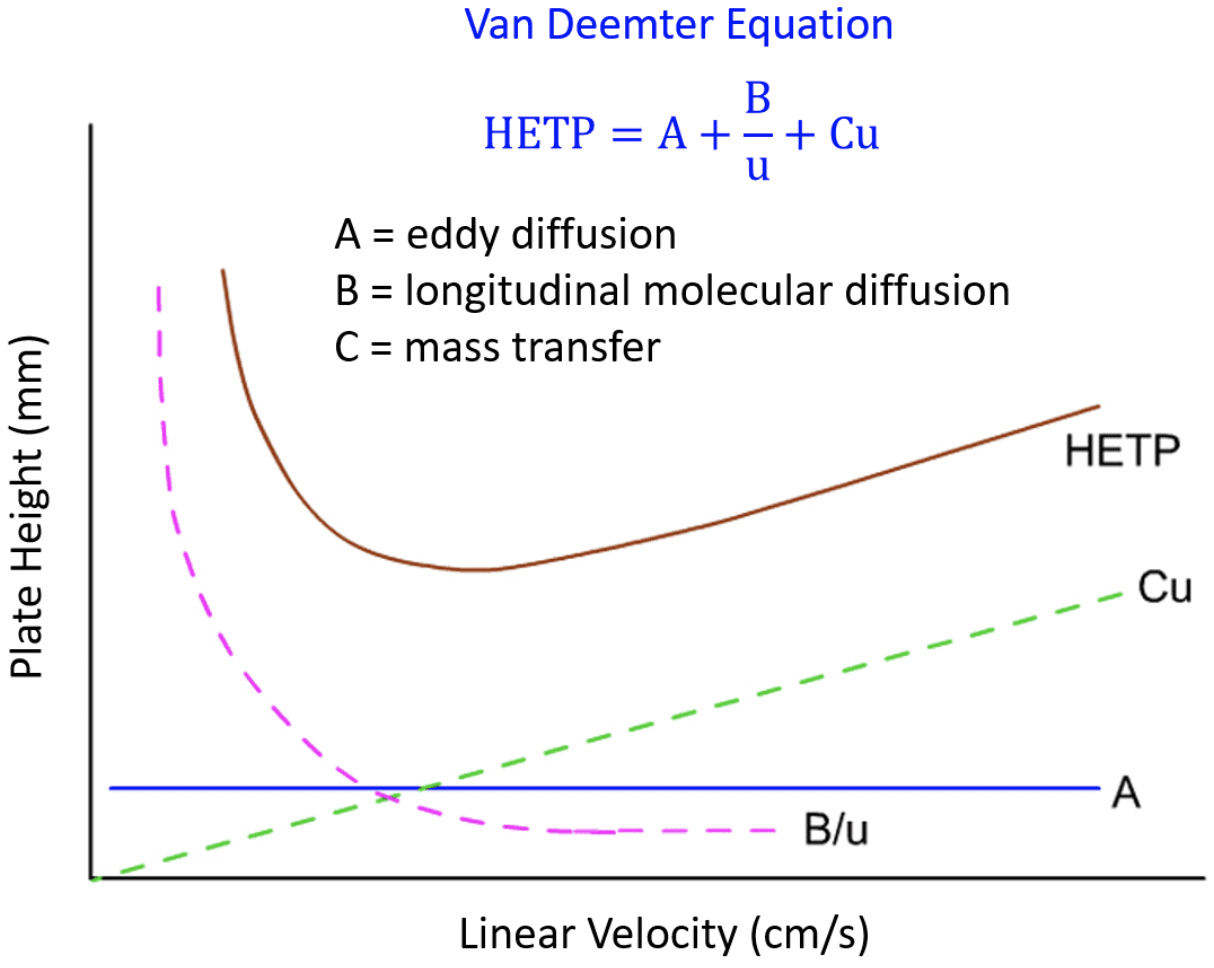
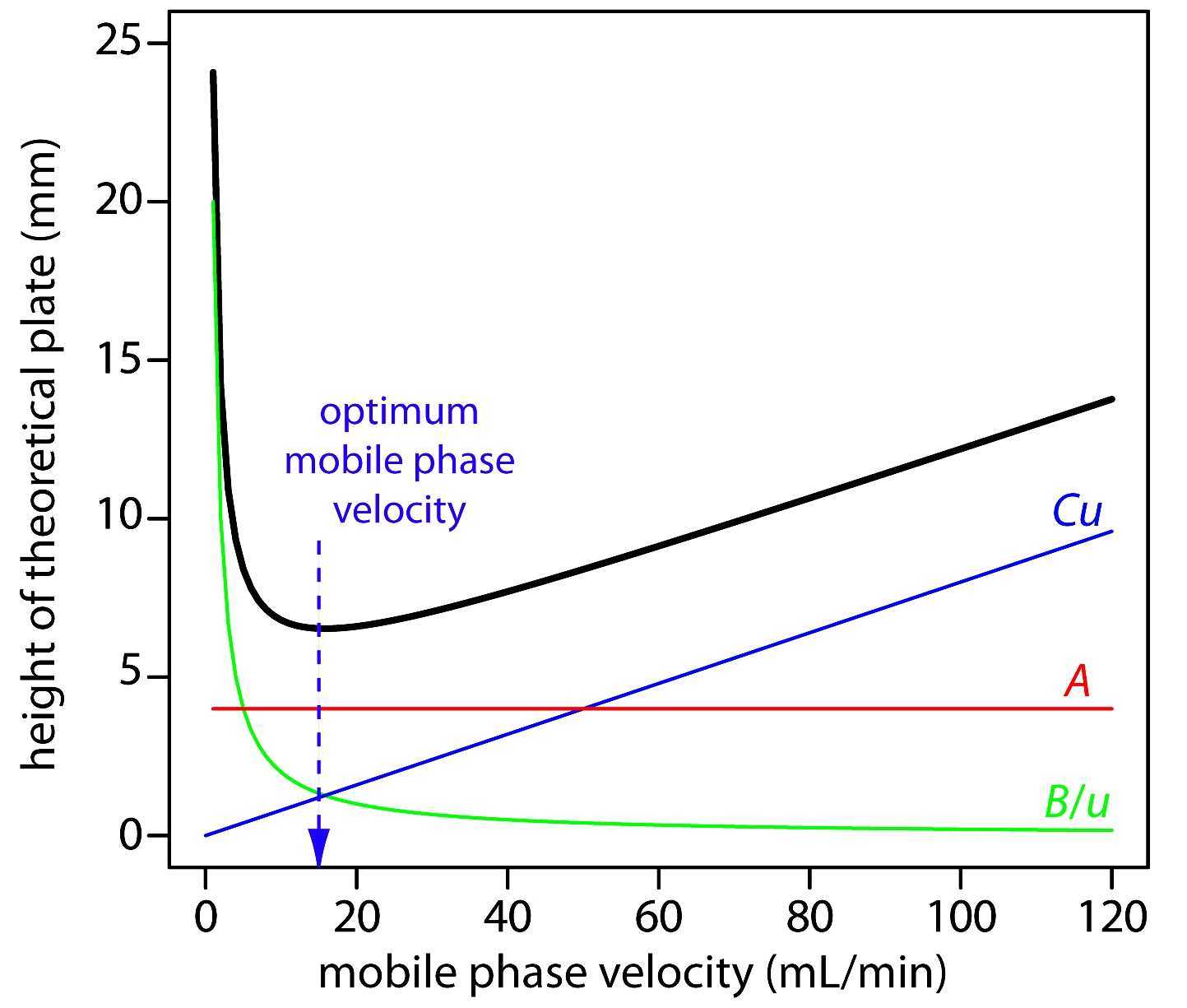
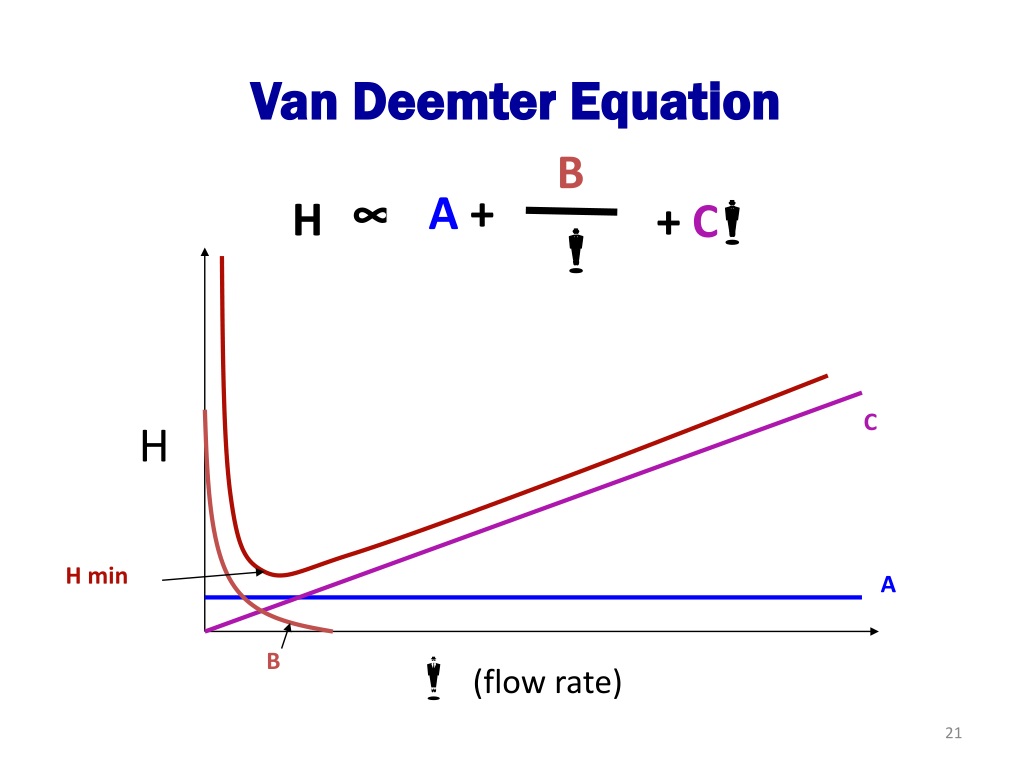
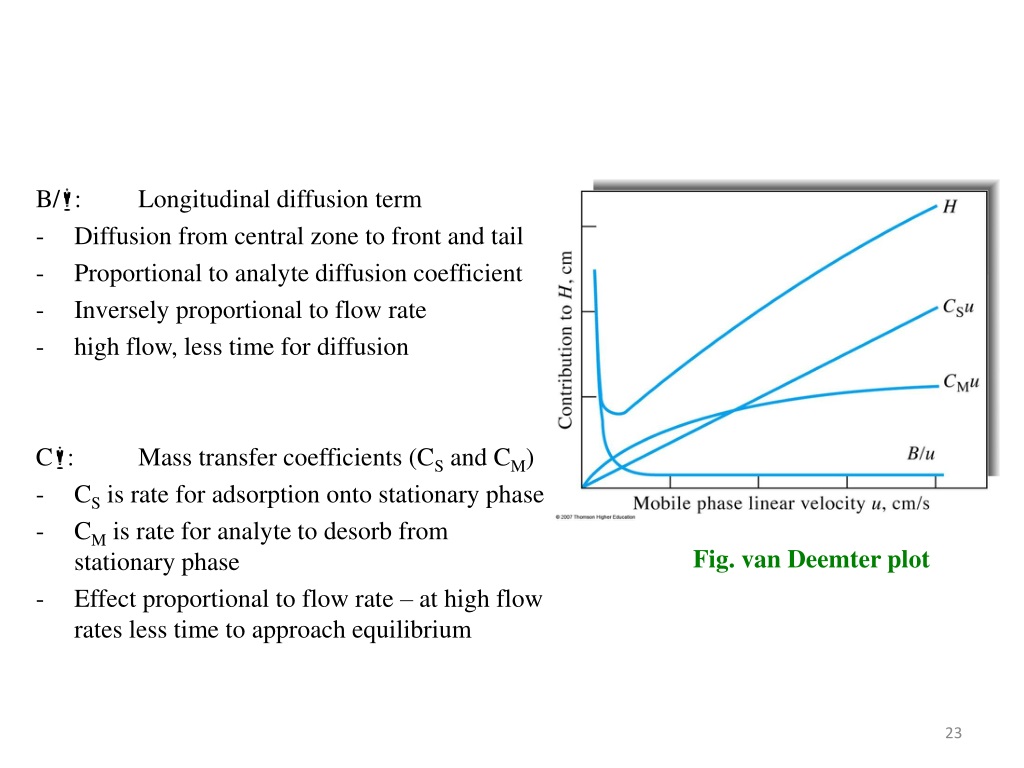
By observing the Van Deemter equation, it can be deduced that an ideal mobile phase flow rate must be determined to yield the best (lowest) value of H. Decreasing the flow rate too much will result in an increase of the longitudinal diffusion factor B/u, while exceedingly increasing the flow rate will increase the significance of the mass transfer term Cu.
PPT Van Deemter Equation PowerPoint Presentation, free download ID9536233
A gas chromatograph (GC) is an analytical instrument that measures the content of various volatile components in a sample. The analysis performed by a gas chromatograph is called gas chromatography.

Van Deemter equation describes that efficiency varies with the linear... Download Scientific
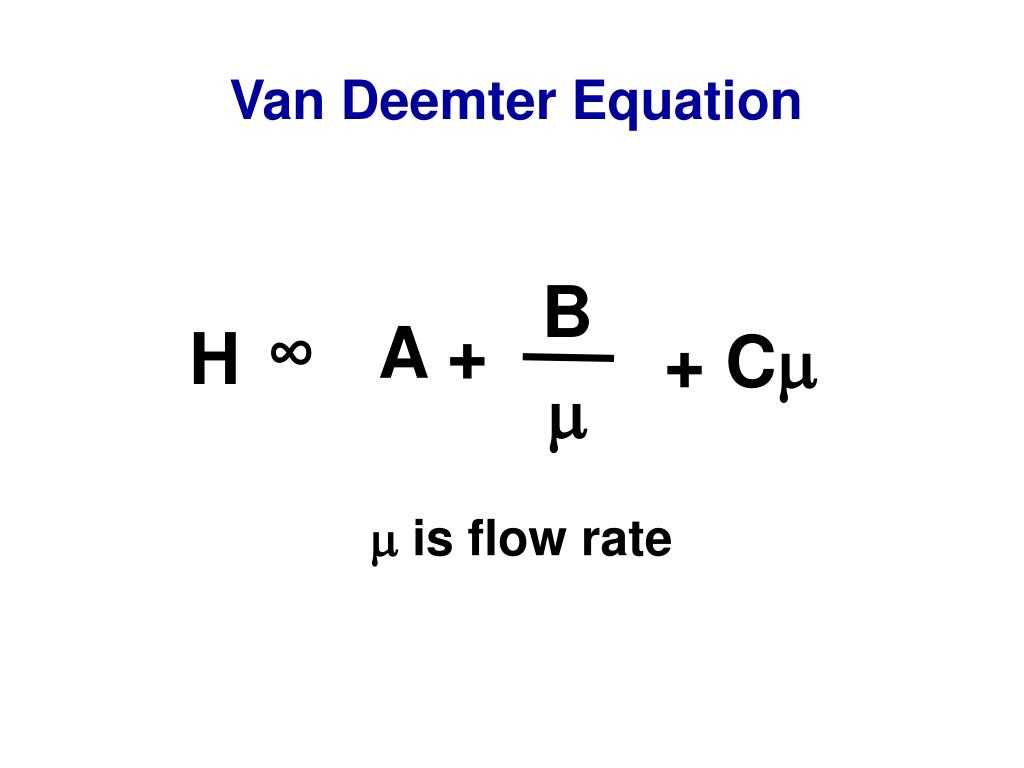
Htot = A + B/u + (CS+ CM)u (For GC, van Deemter equation) Htot = Au1/3+ B/u + (C S+ CM)u (For LC, Knox equation) Basic method for problem solving: 1. Understanding the question. 2. Lay out the parameters regarding this question. 3. Try to use concepts and formulas to find the connections.. Hagen-Poisieulle Equation and Darcy Equation.
PPT Lecture 8 PowerPoint Presentation, free download ID542660
Van Deemter equation Mass transfer resistance in the mobile/stationary phase RPLC and HILIC columns reduced longitudinal diffusion coefficient sample concentration in the mobile phase (mol/m sample concentration in the stationary phase (mol/m reduced overall mass transfer resistance coefficient in the stationary phase
PPT Van Deemter Equation PowerPoint Presentation, free download ID9536233
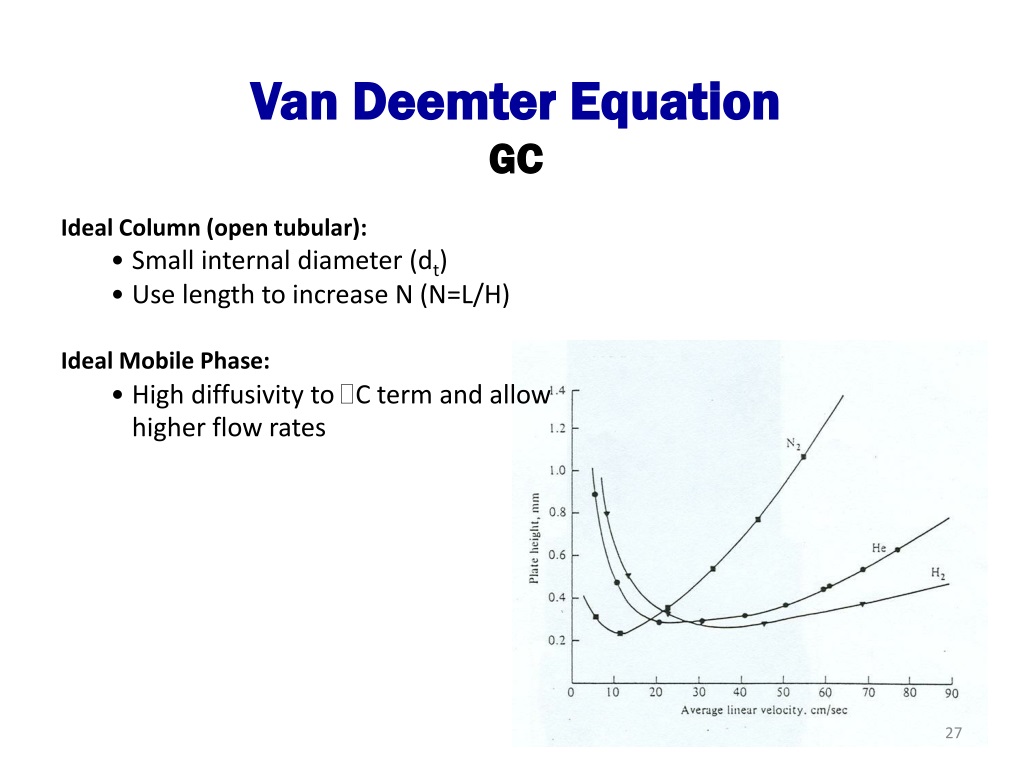
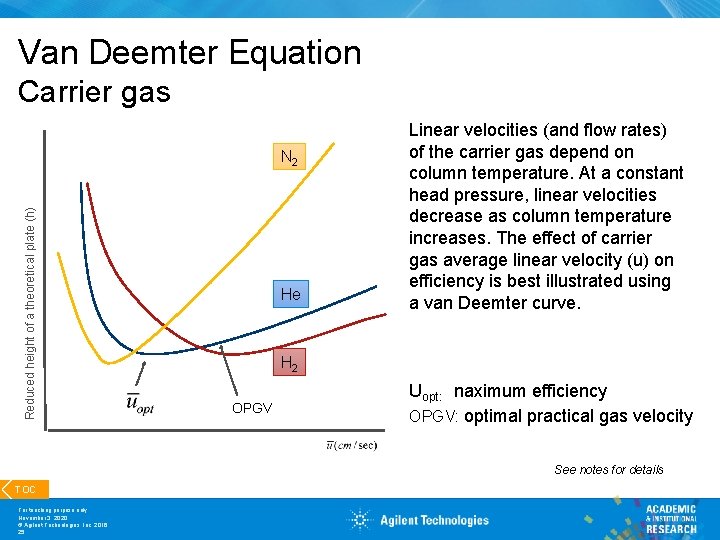
The effect of different carrier gases on column efficiency is represented by the van Deemter (packed columns) and the Golay equation (capillary columns). The van Deemter equation, \ref{2} , describes the three main effects that contribute to band broadening in packed columns and, as a consequence, to a reduced efficiency in the separation process.

Van Deemter equation describes that efficiency varies with the linear... Download Scientific
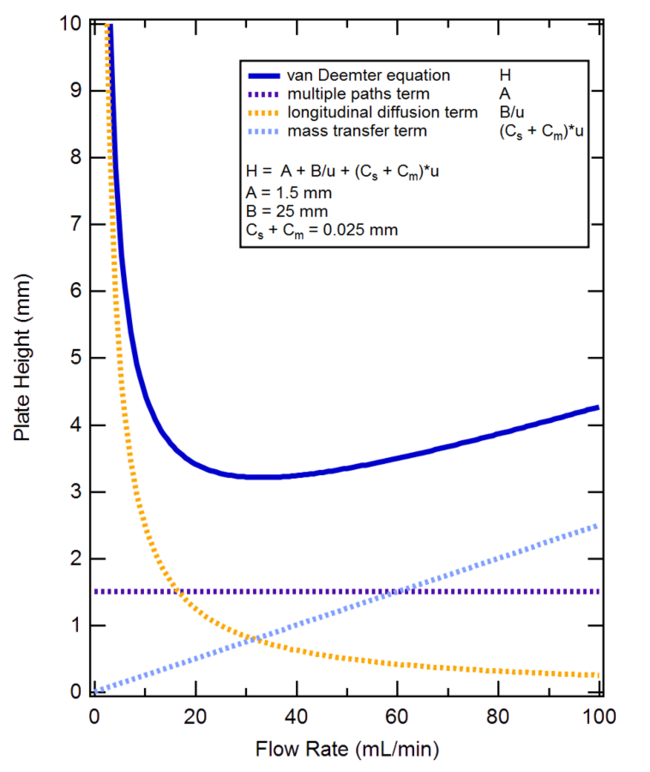
The van Deemter equation is a theoretical treatment of the peak broadening within a chromatographic column. The equation, which describes the band broadening processes, is given by equation (3). (3) h = A + B v + C v where h - reduced plate height, a dimensionless measure of the band broadening. A, B, C are constants.
PPT Van Deemter Equation PowerPoint Presentation, free download ID9536233
Van Deemter equation - The Lockdown guide. Skip to Content. 01357 522 961. Quick Order. My Account. Register. Search. Search. Linear velocity is an important method parameter in chromatography and is affected by changes in flow rate, temperature, column dimensions, mobile phase type and is one of the more important factors t.
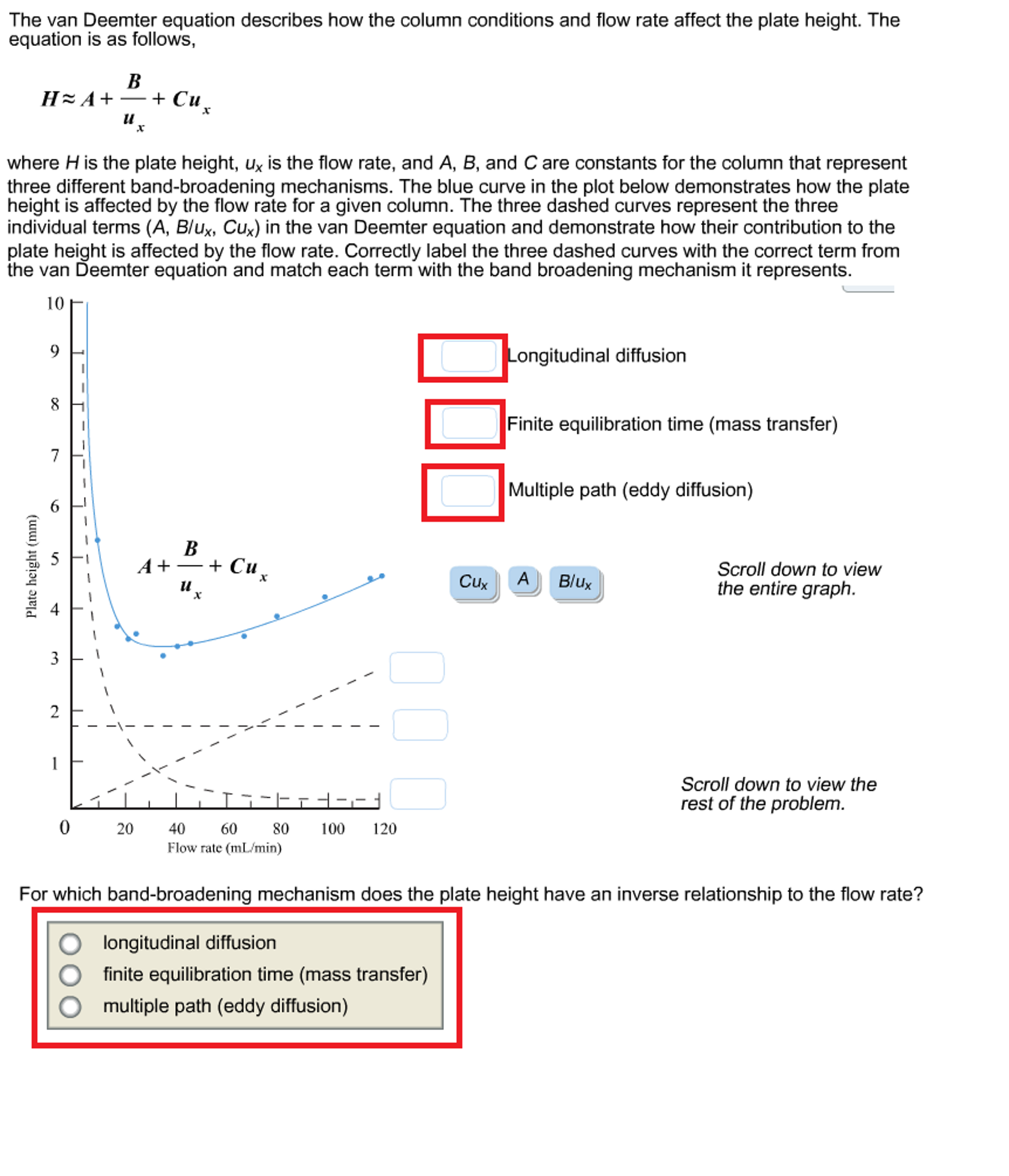
Solved The van Deemter equation describes how the column
Van Deemter equation band broadening helps understand the overall dispersion or widening of a sample peak as it passes through a separation system. The equation signifies the relation between variance per unit length of a separation column to the linear mobile phase velocity in a chromatography setup. The topic explains what is the Van Deemter.
PPT Van Deemter Equation PowerPoint Presentation, free download ID9536233
The Van Deemter equation is a hyperbolic function that predicts that there is an optimum velocity at which there will be the minimum variance per unit column length and, thence, a maximum efficiency. The Van Deemter equation was the result of the first application of rate theory to the chromatography elution process.
PPT Van Deemter Equation PowerPoint Presentation, free download ID9536233
The fundamental mechanisms of band broadening are usually introduced to students through the van Deemter equation. Dimensional analysis of this equation can give physical meaning to the equation coefficients and enhance our understanding relative to qualitative descriptions. This approach can also guide improvements to future liquid chromatography (LC) column designs.
Fundamentals of Gas Chromatography Theory BUILDING BETTER SCIENCE
Access the complete (90 Videos) Analytical Chemistry Video Series here: https://chemguides.com/videos/Access FREE CONTENT here: https://chemguides.com/course.
Vandeemeter equation
The van Deemter equation is a hyperbolic function that predicts that there is an optimum velocity at which there will be the minimum variance per unit column length and, thence, a maximum efficiency. The van Deemter equation was the result of the first application of rate theory to the chromatography elution process. Van Deemter equation
Van Deemter equation HandWiki
The three constants in the van Deemter equation are factors that describe possible causes of band broadening in a particular separation. \(A\) is a constant which represents the different possible paths that can be taken by the analyte through the stationary phase, it decreases if the packing of the column is kept as small as possible.

PPT Ch 21 Principles of Chromatography and Mass Spectrometry Ch 22 Gas and Liquid
As shown in Figure 12.3.7 —which uses the van Deemter equation—the optimum mobile phase velocity is the minimum in a plot of H as a function of u. Figure 12.3.7 . Plot showing the relationship between the height of a theoretical plate, H, and the mobile phase's velocity, u, based on the van Deemter equation.

Solved To The Right Is A Plot Of The Van Deemter Equation...
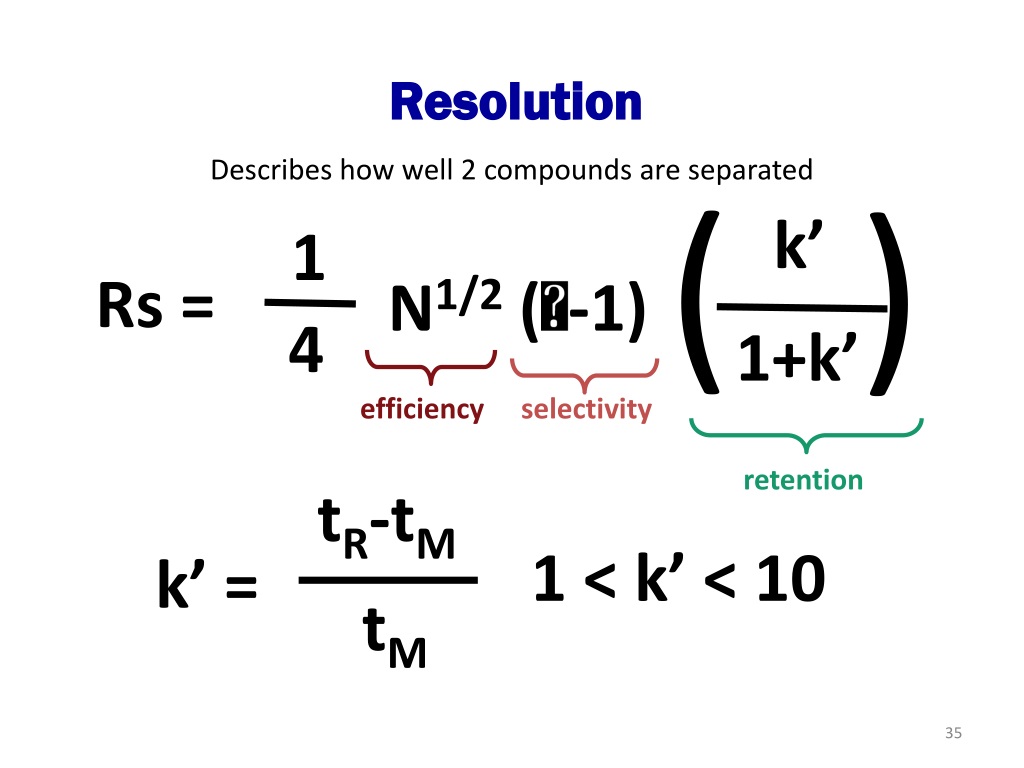
In a simplified form, the Van Deemter equation is: HETP = A + (B / u) + Cu Where: A Eddy diffusion B Longitudinal diffusion C Resistance to mass transfer