Phase Diagram of Water Explanation and Diagrammatic Representation of Phase Diagram of Water

Phase diagram of water. Source... Download Scientific Diagram
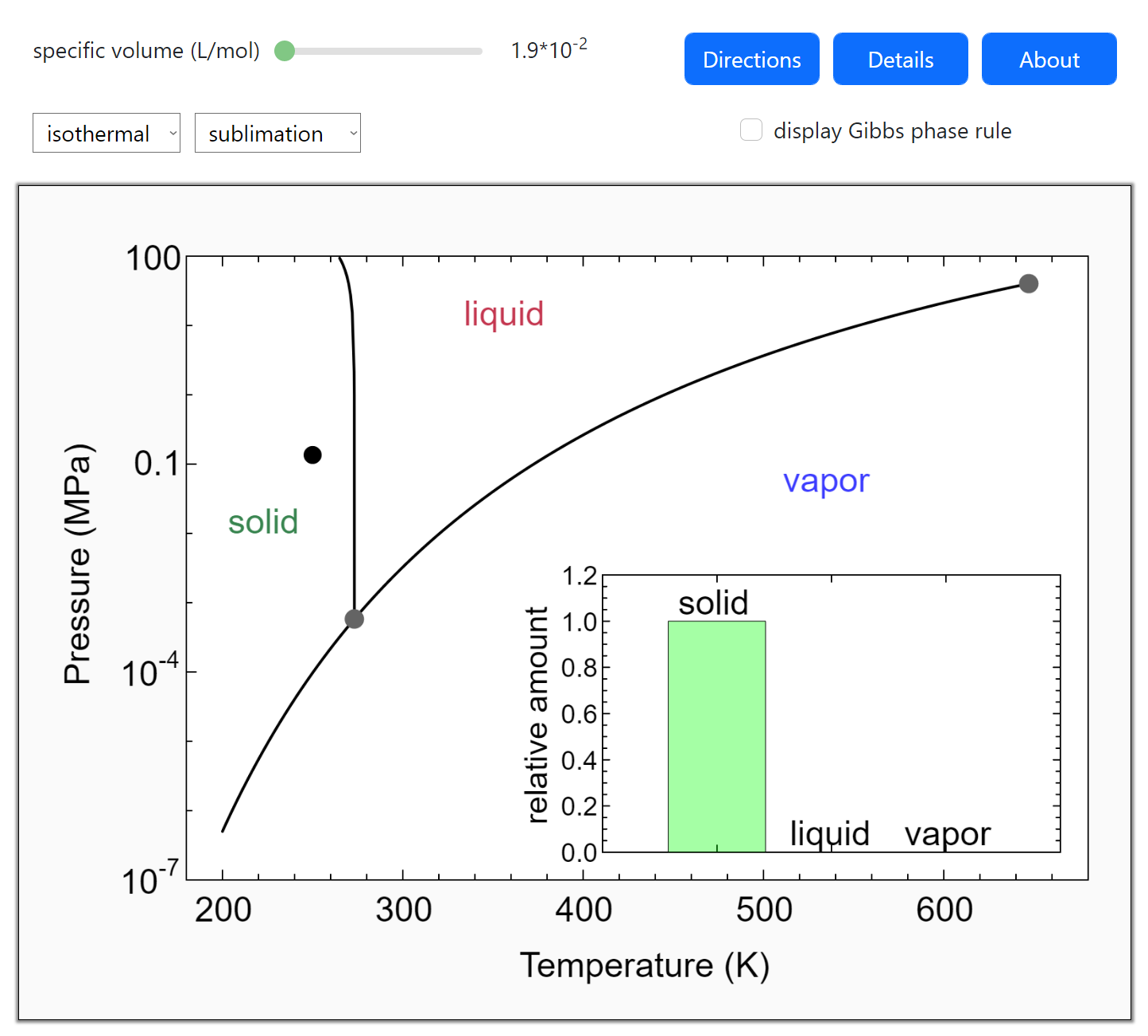
Phase diagram is a graphical representation of the physical states of a substance under different conditions of temperature and pressure. A typical phase diagram has pressure on the y-axis and temperature on the x-axis. As we cross the lines or curves on the phase diagram, a phase change occurs. In addition, two states of the substance coexist.
pressuretemperaturephasediagramforwater LearnChemE
Simplified temperature/pressure phase change diagram for water. The pressure on a pressure-temperature diagram (such as the water phase diagram shown above) is the partial pressure of the substance in question.. A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which.

Schematic water phase diagram. Below the critical point, that is 374 K... Download Scientific
English: Phase diagram of water as a log-lin chart with pressure from 1 Pa to 1 TPa and temperature from 0 K to 660 K, compiled from data in [1] and [2]. Note that the phases of Ice X and XI (hexagonal) differ from the diagram in [3]. The SVG code is valid. This diagram was created with an unknown SVG tool.

Phase Diagram of Water Explanation and Diagrammatic Representation of Phase Diagram of Water
In the video here, Sal uses a horizontal line through the phase diagram. But, it doesn't have to be horizontal. Imagine a vertical line through this diagram-- for water, choose 100 degrees C. As long as you are at 100 C, you can change the phase by changing the pressure on the system.
What is the phase diagram of water? Socratic
The phase diagrams calculated at the classical level with DNN@MB-pol (a) and MB-pol (b), and at the quantum level with MB-pol (c) are compared with the experimental phase diagram (d).The.

Phase Diagrams CK12 Foundation
The phase diagram for water is shown in the figure below. Figure 13.20. 1: Phase diagram for water. Notice one key difference between last section's general phase diagram, and the above phase diagram for water: in water's diagram, the slope of the line between the solid and liquid states is negative rather than positive.
Phase diagram and density of pure water in temperaturepressure space.... Download Scientific
This chemistry video tutorial explains the concepts behind the phase diagram of CO2 / Carbon Dioxide and the phase diagram of water / H2O. This video contai.

—Phase diagram of water Download Scientific Diagram
Consider the phase diagram for carbon dioxide shown in Figure \(\PageIndex{5}\) as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot.
.PNG)
Phase Diagrams Presentation Chemistry
The phase diagram shows that the water would first freeze to form ice as it crossed into the solid area. When the pressure fell low enough, the ice would then sublime to give water vapor. In other words, the change is from liquid to solid to vapor. Example \(\PageIndex{2}\): Phase Diagram for Carbon Dioxide.

Phase Diagram
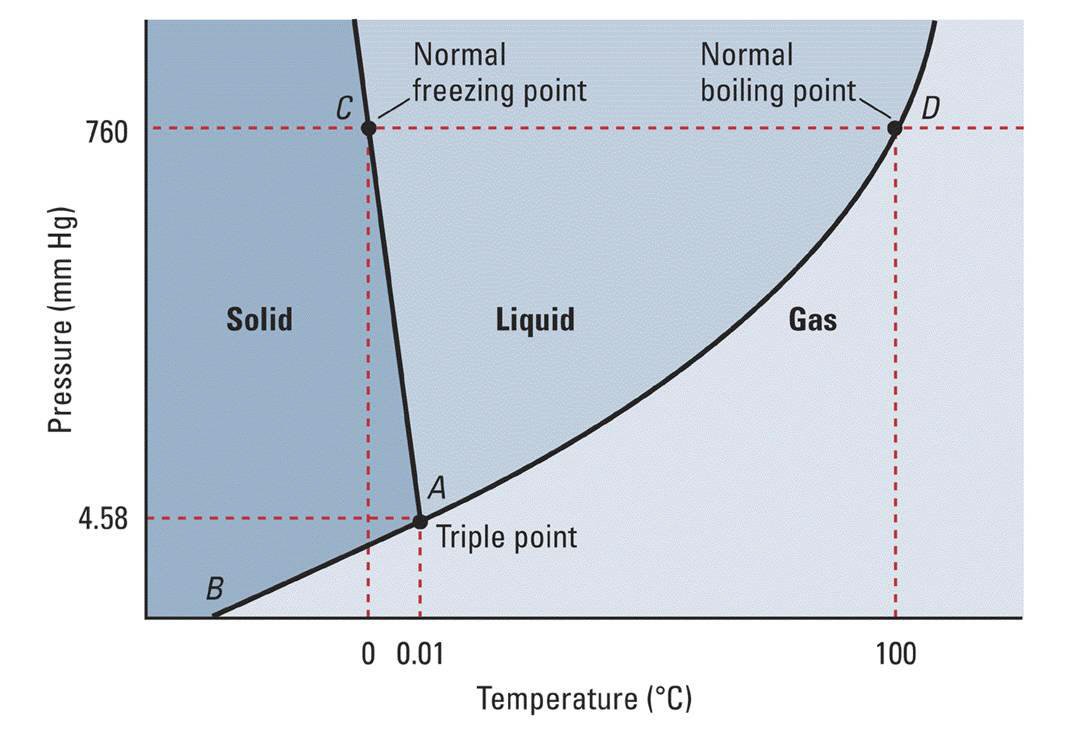
In the phase diagram for water, the variation of the vapor pressure of ice with temperature is shown by the line AT. As might be expected, the vapor pressure of ice is quite small, never rising above 0.006 atm (0.61 kPa). The vapor pressure of liquid water is usually much higher, as is shown by the curve TC. The point C on this curve.

physical chemistry Why does increase in pressure favor formation of water in waterice
We can use the phase diagram to identify the physical state of a sample of water under specified conditions of pressure and temperature. For example, a pressure of 50 kPa and a temperature of −10 °C correspond to the region of the diagram labeled "ice.". Under these conditions, water exists only as a solid (ice).

The phase diagram of water ((© ChemWiki (© UC Davis, Wikimedia Commons) Download Scientific
Phase diagram for ammonium nitrate and water binary mixture. Above the blue curve is the liquid state, while below is the solid state. Crystals and Polymorphs. Crystalline solids can have multiple possible structures, dependent on thermodynamic variables. This is why you may hear someone talk about different forms of ice, such as ice II, ice.

thermodynamics Vacuum freezing of water Physics Stack Exchange
An Efficient Way to Predict Water's Phases. A machine-learning technique maps water's phase space as reliably as gold standard ab initio calculations but at a much smaller computational cost. Given its familiarity, water is a surprisingly tricky substance to simulate. In addition to having liquid and gas phases, water adopts at least 14.

phase Thermodynamics of SolidLiquid Chemistry Stack Exchange
Depiction in Phase Diagram of Water. At different temperatures and pressures, a phase diagram depicts the preferred physical states of matter. Water is a liquid at typical room temperatures and pressures, but it becomes solid (i.e. ice) if the temperature is reduced below 273 K, and gaseous (i.e. steam) if the temperature is increased above 373 K at the same pressure.

Phase Diagram of Water (H2O)
Here, we compute the phase diagram of water at three hybrid DFT levels of theory (revPBE0-D3, PBE0-D3 and B3LYP-D3), accounting for thermal and nuclear fluctuations as well as proton disorder. We.

Phase Diagrams of Water 101 Diagrams
The phase diagram for water is shown in the Figure below . Figure 13.26. Phase diagram for water. Notice one key difference between the general phase diagram and the phase diagram for water. In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual.