Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube

Solved Experiment (?)B Standardization of HCl with Na2CO3
The balanced equation for the chemical reaction of Na₂CO₃ and HCl is given as: Na₂CO₃ (aq) + 2 HCl (aq) → 2 NaCl (aq) + CO₂ (g) + H₂O (l) The total ionic equation is the equation demonstrating the dissociation of the reactant and products into their respective ions. The total ionic equation for this reaction is:

Na2CO3 vs HCl Titration CBSE Chemistry Practical Titration Class 11 Bhatia Mam Classes YouTube
Write a balanced net ionic equation for the reaction of Na2CO3(s) and HCl(aq). A) Na2CO3(s) + 2 HCl(aq) → 2 NaCl(aq) + H2O(1) + CO2(g) B) 2Na+(aq) + CO32-(aq) + 2H.
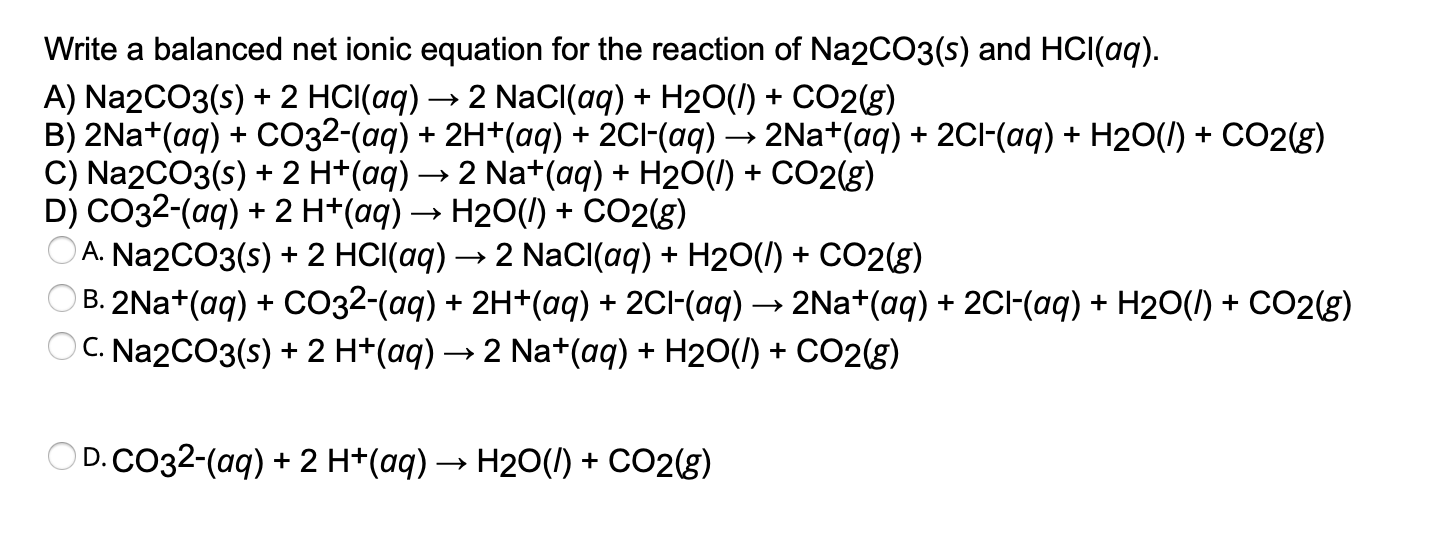
Solved Write a balanced net ionic equation for the reaction
This chemistry video explains how to write the balanced molecular equation and the net ionic equation of the reaction between Sodium Carbonate and Hydrochloric Acid. It also explains how to.

Net Ionic Equation for BaCl2 + Na2CO3 = BaCO3 + NaCl YouTube
The unbalance equation is: HCl + Na2CO3 = NaCl + H2O + CO2 Following steps are applied to equate the above-mentioned reaction: For the reaction to be balanced, the number of atoms of each element present on both the reactant and product sides must be equal. Table representing count of atoms from the unbalanced equation
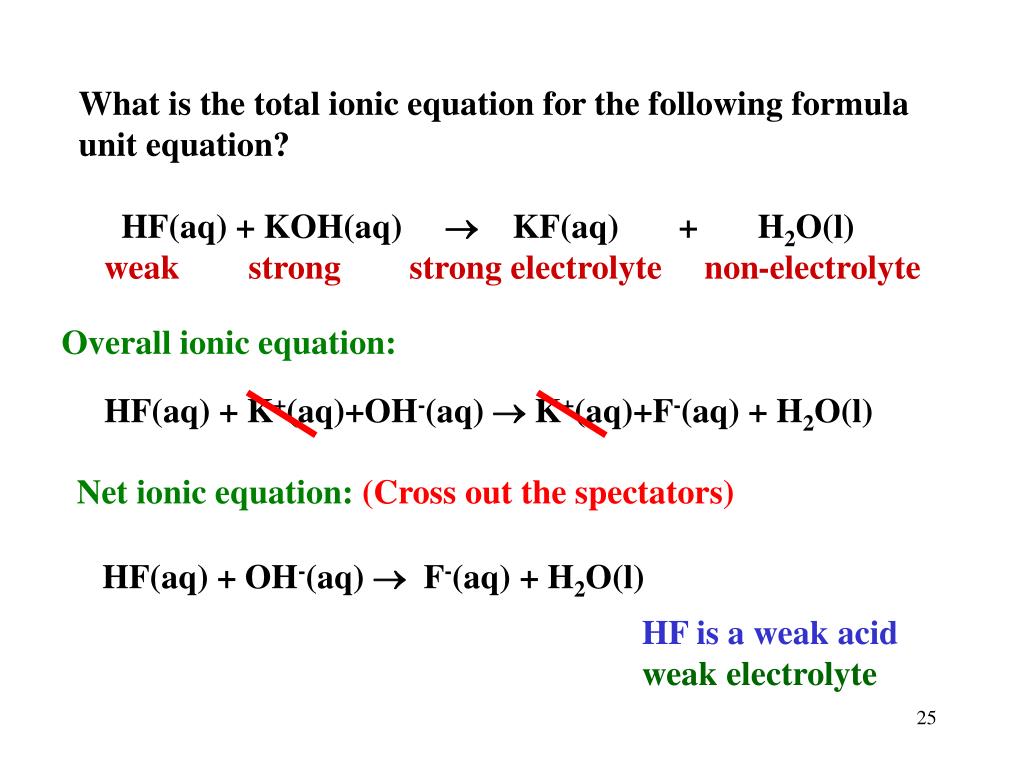
PPT Ionic Equations.... PowerPoint Presentation, free download ID6673081
Get the free "NET IONIC EQUATION CALCULATOR" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

SOLVED Wrrite the net ionic equation for the following reaction Na2CO3 (aq)+ 2HCl (aq)→CO2 (g
0:00 / 3:11 Net Ionic Equation for Na2CO3 + HCl | Sodium Carbonate + Hydrochloric Acid Geometry of Molecules 1.28K subscribers Subscribe 254 views 10 months ago Explanation Hello, Chemistry.

Solved Experiment (?)B Standardization of HCl with Na2CO3
This is the equation given by my textbook for hydrolysis of sodium carbonate: NaX2COX3 +2HX2O HX2COX3 +2NaX+ +2OHX− N a X 2 C O X 3 + 2 H X 2 O H X 2 C O X 3 + 2 N a X + + 2 O H X −. and it mentions that sodium ion (NaX+) ( N a X +) does not tend to combine with the hydroxide ion (OHX−) ( O H X −) and I was wondering what prevents them.

Net Ionic Equation and Complete Ionic Equation
To write the net ionic equation for Na2CO3 + HCl we follow three steps. First, we balance the molecular equation. Second, we break the soluble ionic compounds, the ones with an (aq) after.

How to balance Na2CO3+HCl=NaCl+CO2+H2OChemical equation Na2CO3+HCl=NaCl+CO2+H2ONa2CO3+HCl
Ag + ( a q) + Cl − ( a q) → AgCl ( s) This net ionic equation tells us that solid silver chloride is produced from dissolved Ag + and Cl − ions, regardless of the source of these ions. In comparison, the complete ionic equation tells us about all of the ions present in solution during the reaction, and the molecular equation tells us.
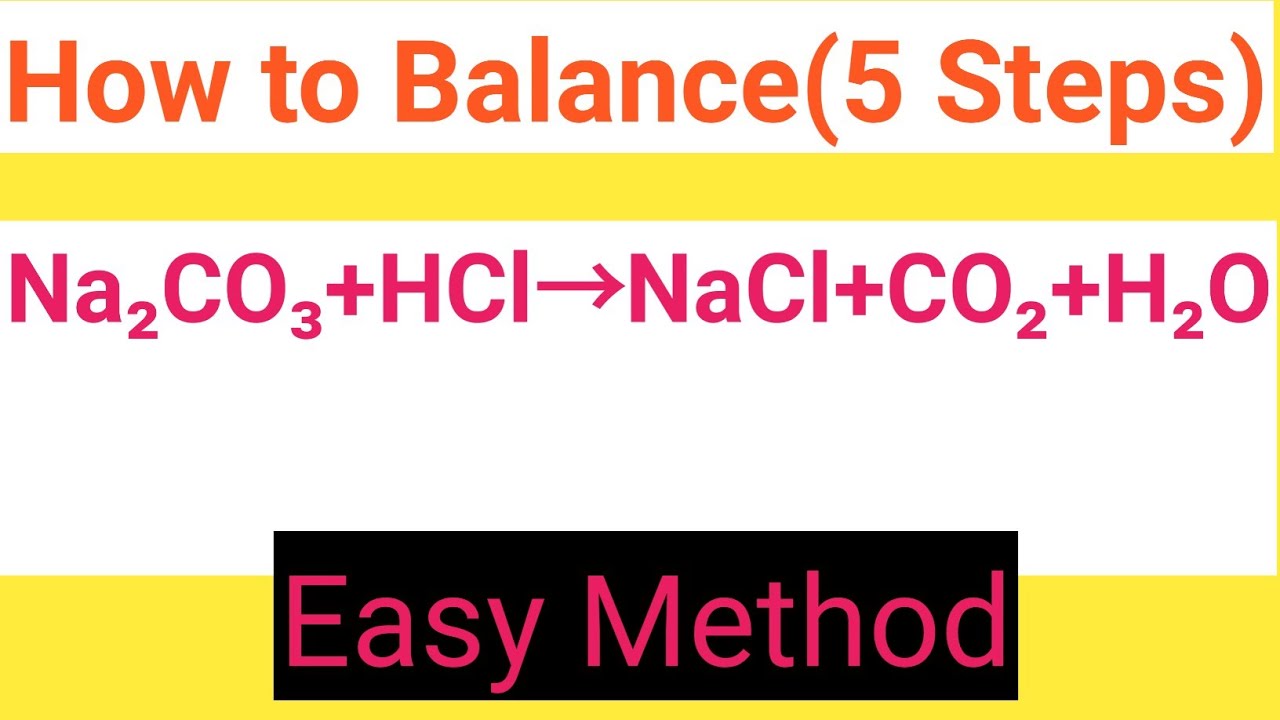
Na2CO3+HCl=NaCl+CO2+H2O Balanced EquationSodium carbonate+Hydrochloric acid=Sodium chloride
HCl + Na2CO3 = NaCl + H2O + CO2 - Balanced Chemical Equation HCl + Na2CO3 = NaCl + H2O + CO2 - Balanced Chemical Equation Balanced Chemical Equation 2 HCl + Na 2 CO 3 → 2 NaCl + H 2 O + CO 2 ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information Word Equation
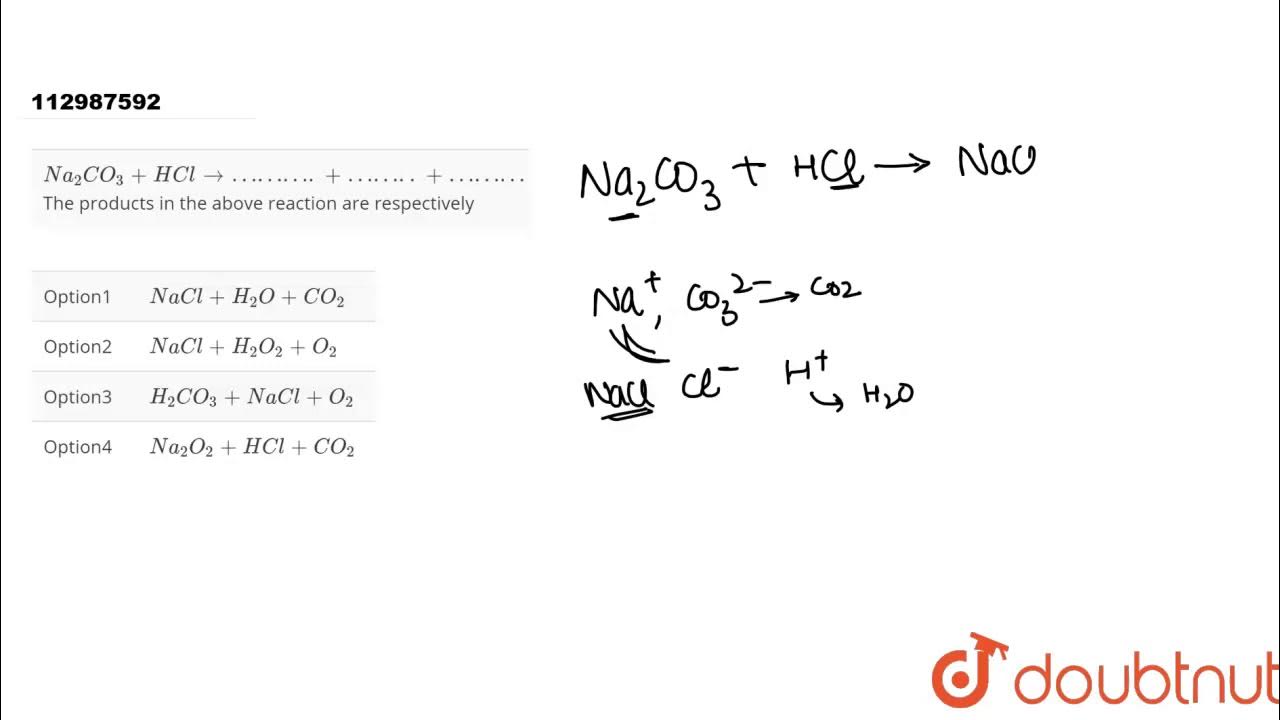
`Na_(2)CO_(3)+HCl to ………. + …….. + ………` The products in the above reaction are respectively
To write the net ionic equation for the reaction between Na2CO3 and HCl, we first need to write the balanced molecular equation: Na2CO3 + 2HCl -> 2NaCl + H2O + CO2. Next, we can identify the spectator ions , which are ions that do not participate in the chemical reaction.

27) Give the net ionic equation for the reaction (if any) that Loccurs when aqueous solutions
Give the net ionic equation for the reaction (if any) that occurs when aqueous solutions of Na2CO3 and HCl are mixed. Group of answer choices 2 Na+ (aq) + CO32- (aq) + 2 H+ (aq) + 2 Cl- (aq) → H2CO3 (s) + 2 Na+ (aq) + 2 Cl- (aq) 2 H+ (aq) + CO32- (aq) → H2O (l) + CO2 (g) 2 Na+ (aq) + CO32- (aq) + 2 H+ (aq) + 2 Cl- (aq) → H2CO3 (s) + 2 NaCl (aq)
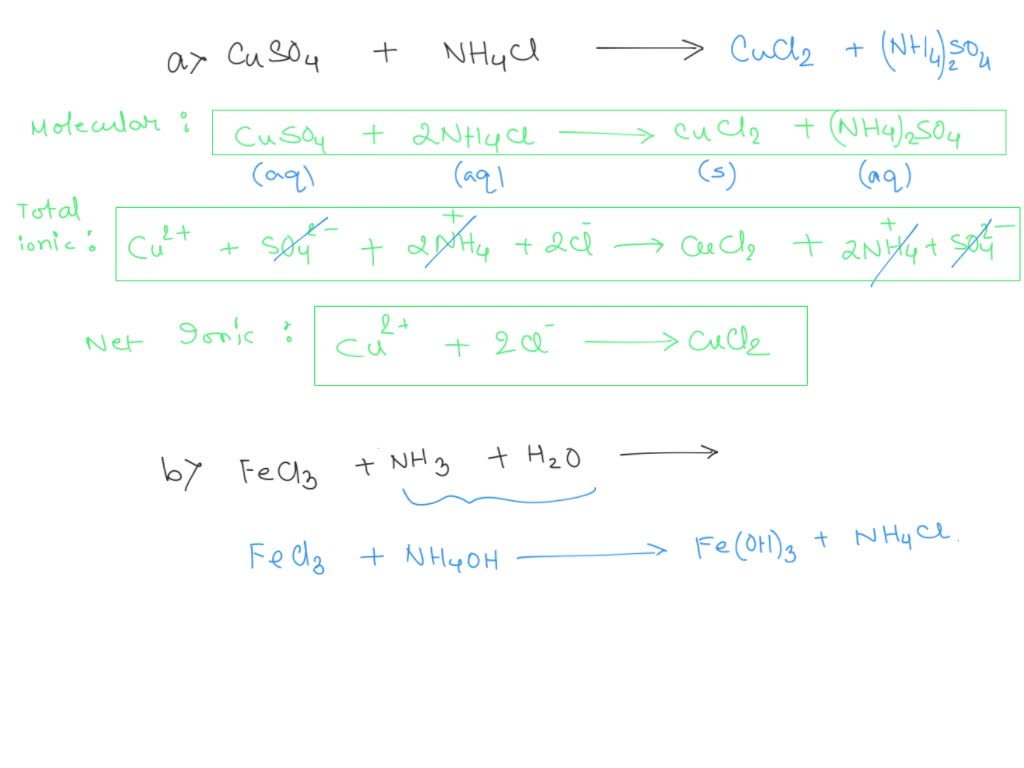
SOLVED write equations (molecular, total ionic, net ionic) for the reaction of CuSO4 and Na2CO3
The dissolving equation is Na 2 SO 4 (s) → 2Na + (aq) + SO 4 2 − (aq) Exercise 8.11.1 8.11. 1. Write the chemical equation that represents the dissociation of (NH 4) 2 S. Answer. When chemicals in solution react, the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions, not the.
[Solved] Hydrochloric acid (HCl) reacts with sodium carbonate (Na2CO3),... Course Hero
One mole of Sodium Carbonate [Na 2 CO 3] and two moles of Hydrogen Chloride [HCl] react to form two moles of Sodium Chloride [NaCl], one mole of Water [H 2 O] and one mole of Carbon Dioxide [CO 2] Show Chemical Structure Image for HCl into tubes containing Na2CO3 → Cavitation caused by carbon dioxide (CO2) produced
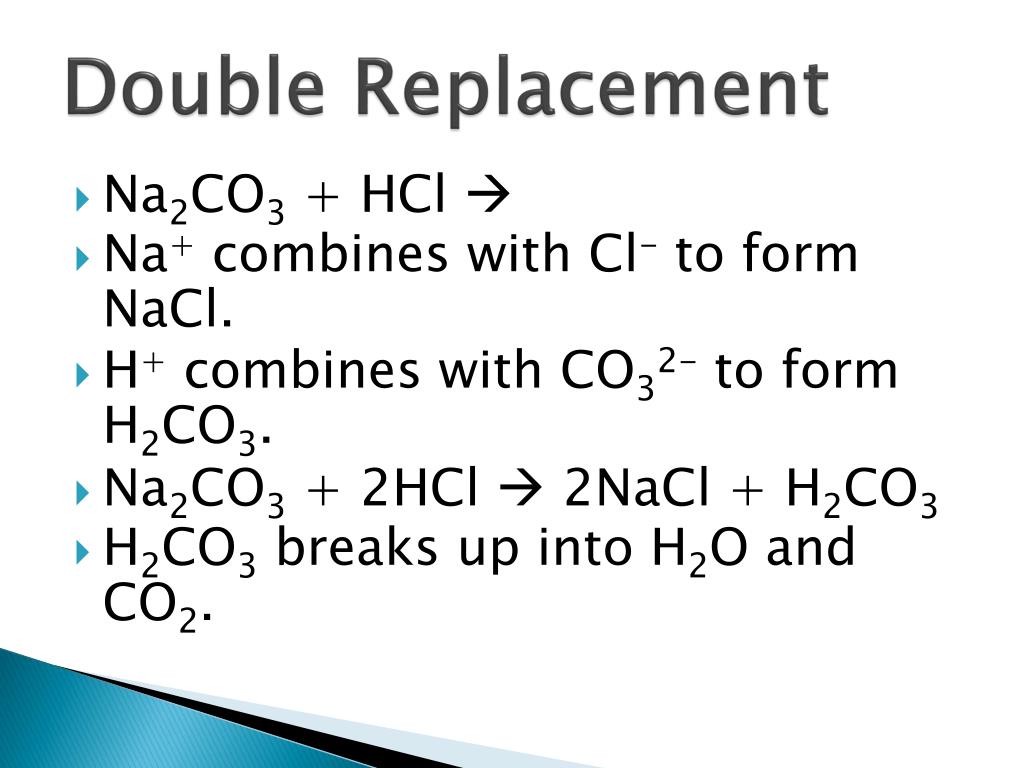
PPT Writing Chemical Reactions PowerPoint Presentation, free download ID2132791
This chemistry video tutorial explains how to predict the products of the reaction between Sodium Carbonate and Hydrochloric Acid. It explains how to write.

How to Write the Net Ionic Equation for Na2CO3 + Sr(NO3)2 = NaNO3 + SrCO3 YouTube
Neutralization Reactions and Net Ionic Equations for Neutralization Reactions. A neutralization reaction is a reaction in which an acid and a base react in an aqueous solution to produce a salt and water. The aqueous sodium chloride that is produced in the reaction is called a salt.