Mg Oh 2 Hcl Compuesto

In this video, we are going to share the process for naming the polyatomic chemical compounds.
Calculate the molecular mass of the following:Mg(HCO3)2 (Magnesium Bicarbonate)SUBSCRIBE if you'd like to help us out!https://www.youtube.com/channel/UC2C34W.

What is the correct increasing order of solubility of the following salt? NaHCO3, KHCO3, Mg(HCO3
Write the names of the following compounds and identify each as ionic or covalent compound: Mg(HCO3)2 _____ CO _____ P4 _____ SiS2 _____ PBr3 CHAPTER 6: Compounds and their Bonds 1. Write the names of the following compounds and identify each as ionic or covalent compound:

(i) Boiling During bour, g, the soluble Mg(HCO3 )2 is converted int in...
Write the names or chemical formulas of given compounds; Mg(HCO3)2, KI, Iron(III)chloride, nitric acid, BH3, Carbondioxide. This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.

Mg(HCO3)2和Ba(OH)2如何反应_百度知道
In this video we'll write the correct name for Mg(HCO3)2. To write the name for Mg(HCO3)2 we'll use the Periodic Table and follow some simple rules.Because M.
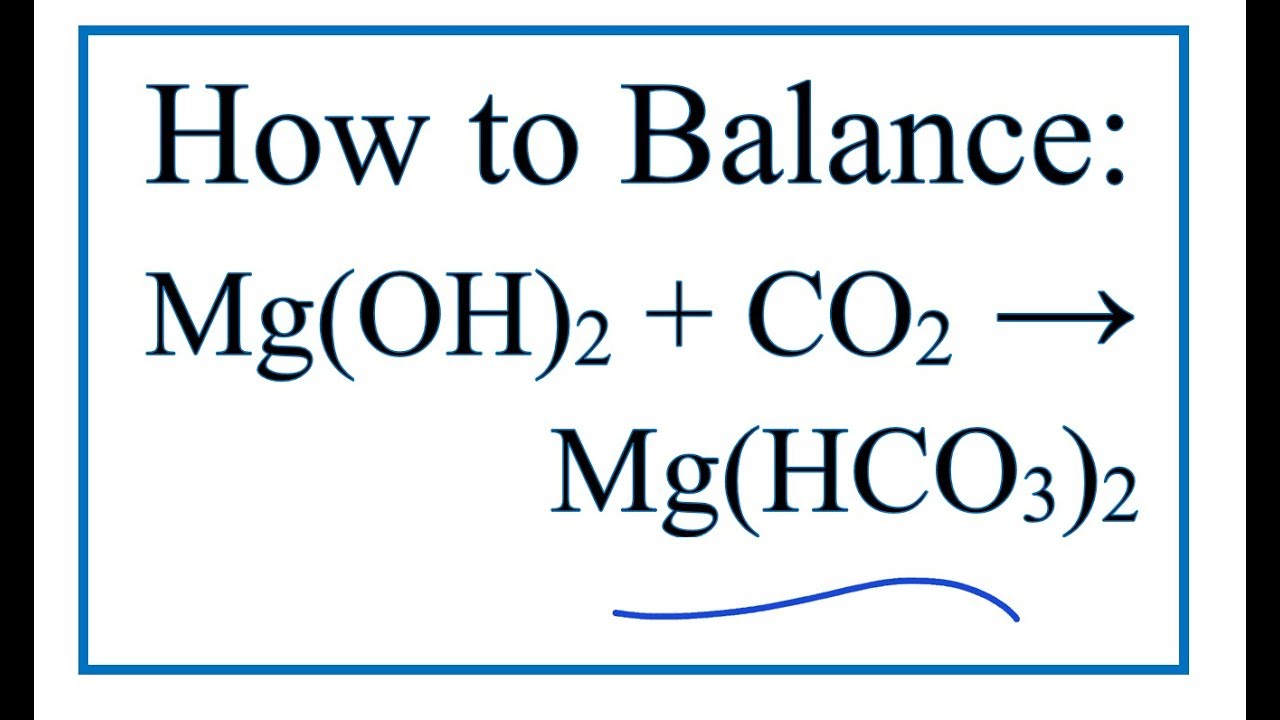
Mg Oh 2 Hcl Compuesto
Common Cations and Anions Name Formula Charge Name Formula Charge Name Formula Charge aluminum Al 3+ +3 magnesium Mg 2+ +2 carbonate CO 3 2- -2 ammonium NH 4 + +1 manganese (II) Mn 2+ +2 chlorate ClO 3 - -1 barium Ba 2+ +2 manganese (III) Mn 3+ +3 chloride Cl - -1 cadmium Cd 2+ +2 mercury (I)

Which of the following statements regarding the property of hard water is/are correct?1
Magnesium Hydrogen Carbonate Name: Magnesium Hydrogen Carbonate Alias: Magnesium Bicarbonate Formula: Mg (HCO3)2 Molar Mass: 146.3387 Mg (HCO3)2 Molar Mass Converter Weight: Mole: Mg (HCO3)2 is a white powder at room temperature. It is soluble in water. If the concentration of Mg (HCO3)2 or Ca (HCO3)2 is too high, the water is called hard water.
[Solved] What is the name of this compound shown below. The systematic name... Course Hero
Magnesium bicarbonate PubChem CID 102204 Structure Molecular Formula Mg(HCO3)2 C2H2MgO6 Synonyms Magnesium bicarbonate 2090-64-4 Magnesium bis (hydrogen carbonate) magnesium hydrogen carbonate magnesium;hydrogen carbonate View More. Molecular Weight 146.34 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound

How to Write the Name for Mg(HCO3)2 YouTube
Steps to calculate molar mass Identify the compound: write down the chemical formula of the compound. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Find atomic masses: look up the atomic masses of each element present in the compound.

Solution Name of the hardness Amount of Physical Chemistry
Magnesium Bicarbonate. Magnesium bicarbonate, also known by its IUPAC name magnesium hydrogen carbonate, is a salt containing bicarbonate anion and magnesium cation represented by the formula C 2 H 2 MgO 6 or Mg (HCO 3) 2 [1]. Since magnesium bicarbonate is unstable in a solid state, it exists in a dilute aqueous solution [2].

Nêu kết quả sau khi trộn lẫn 5 dung dịch NaHSO4, NaCl, Mg(HCO3)2, Na2CO3, Ba(HCO3)2 với nhau
a. FeCl3 iron chloride b. NO2, nitrogen (IV) oxide c. CaO, calcium (II) monoxide d. Al2S3, dialuminum trisulfide e. Mg (C2,H3O2)2, manganese diacetate f. FePO4, iron (II) phosphide g. P2S5, phosphorus sulfide h.
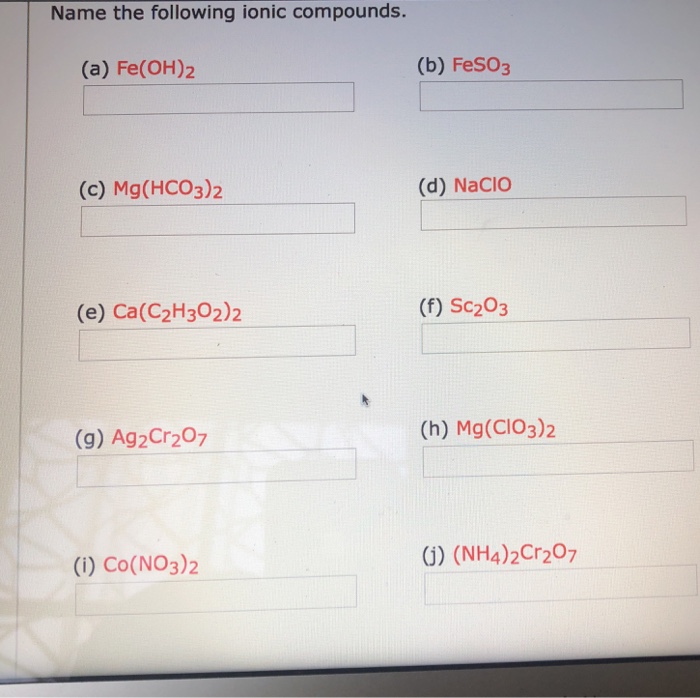
Solved Name the following ionic compounds. (a) Fe(OH)2 (b)
The first step to finding the molar mass of Magnesium Bicarbonate is to count the number of each atom present in a single molecule using the chemical formula, Mg (HCO3)2: 2. Find Atomic Mass of Each Element Next, using the periodic table, find the atomic mass in g/mol of each element (the molar mass of an element is equal to its atomic mass): 3.
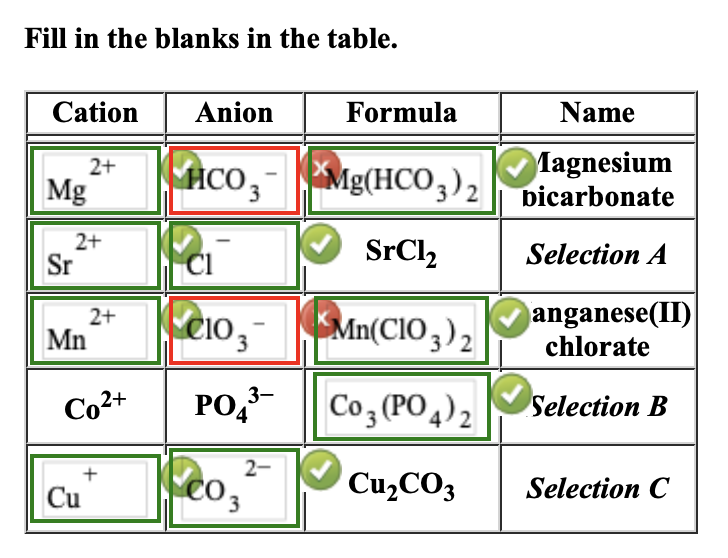
Solved Fill in the blanks in the table. Cation Anion Formula
The systematic name of Mg (HCO3)2 is magnesium carbonate O magnesium bicarbonate magnesium formate O magnesium acetate 111 This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer

Temporary hardness of water is due to the presence of
Question: Name the following ionic compounds. (a) Fe(OH)2 (b) FeSO3 (c) Mg(HCO3)2 (d) NaCIO (e) Ca(C2H302)2 (f) Sc203 (g) Ag2Cr207 (h) Mg(CIO3)2 (i) Co(NO3)2 ) (NH4)2Cr207 . Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We reviewed their content and use your.

SOLVED A sample of water on analysis has been found to contain the following in ppm Ca(HCO3)2
Compute answers using Wolfram's breakthrough technology & knowledgebase, relied on by millions of students & professionals. For math, science, nutrition, history.

What is the correct increasing order of solubility of the following salt? NaHCO3, KHCO3, Mg(HCO3
Q1 What is the difference between magnesium carbonate and magnesium bicarbonate? Ans: The chemical formula of magnesium carbonate is MgCO 3 and magnesium bicarbonate is Mg (HCO 3) 2 . The magnesium carbonate is an inorganic salt, a white solid. Whereas magnesium bicarbonate exists in an aqueous solution. Q2

molecular weight of mg(Hco3) 2 explain Brainly.in
1 Answer Sorted by: 2 Typically dissociation refers to the ions that are formed when a substance dissolves. The most common solvent is water. So in aqueous solution: Mg(HCOX3)X2(s) MgX2+ +2HCOX3X− M g ( H C O X 3) X 2 ( s) M g X 2 + + 2 H C O X 3 X − The bicarbonate will also form very minor amounts of other aqueous species: