Valency of an element, structure of the atom, class 9 science, chemistry YouTube

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio How to tell how many
The valency of the first 30 elements of the periodic table is given below. Periodic Trends in the Oxidation States of Elements 1. Variation Of Oxidation State Along a Period While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8.
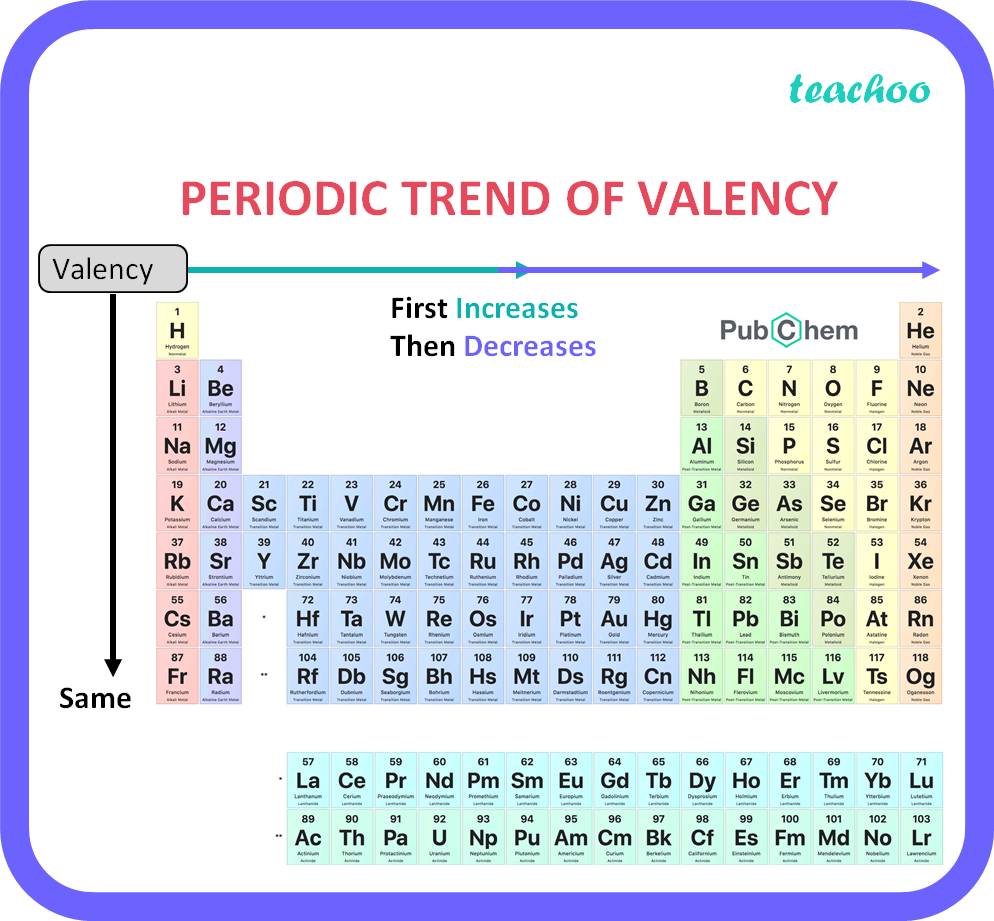
How does valency of an element vary across a period Class 10 Teachoo
The number of electrons that should be lost or gained to achieve this stable configuration is known as valency . Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class Book a free demo Next: What is Atomicity? Important → Ask a doubt Class 9 Chapter 3 Class 9 - Atoms And Molecules Tired of ads?

Science 9 Chapter 3 Ions, Polyatomic ions Examples of Some Cations and Anions with valency
Element Valency PDF. The outer shell of a fluorine atom contains 7 electrons. This means it has one less electron than needed to complete the shell. This gives fluorine a -1 valence. This element Valency PDF is a downloadable version of the Valences of the Elements table. As in the table, the most common valences are in BOLD text where values.

Class 9 Periodic Table Of Elements With Atomic Mass And Valency Periodic Table Timeline
172 Similar questions Q. An element A belongs to the third period and group 1 of the periodic table. Find out : i. The number of valence electrons in its atoms. ii. Valency of the element. iii. Name of the element. Q. The valency of elements in the periodic table with respect to hydrogen:

valency table for class 9 Brainly.in
How to calculate Valency https://youtu.be/Bb3cZNefadEElectronic Configuration https://youtu.be/L7Qu6dYe6ewHow to write valency. How to write Electronic confi.

Valency
Structure of atom of Class 9 We have studied earlier about the arrangement of electrons in different shells/orbits. "The electrons present in the outermost shell/orbit of the atom of an element are called valence electrons." In all chemical reactions, only the electrons present in outermost orbit will take part in the reaction.

What is Valency Explain with Example Valency Definition Trick to Learn Valency of Elements
The valency chart is a table that shows how many bonds an atom can form with other atoms. The table is arranged so that each row corresponds to an element, and each column corresponds to a valence level. When reading the valency chart, it is important to remember that atoms can only form as many bonds as they have electrons in their outer shell.
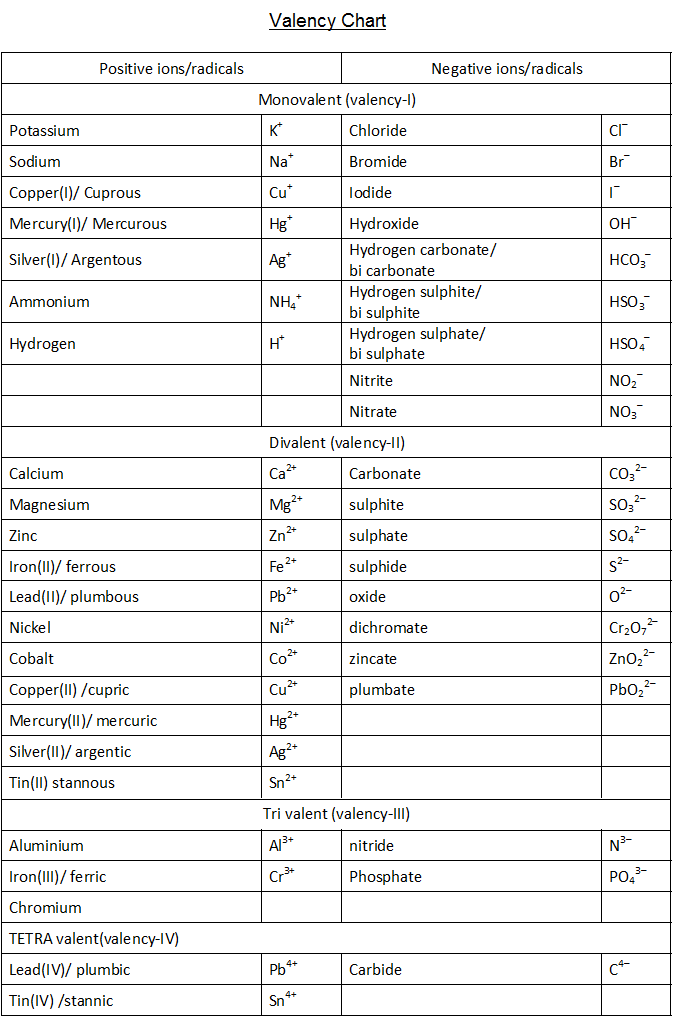
VALENCY CHART
Understand the concept of #valency in different elements and how to find it, with examples.FREE Registration: http://deltastep.com or install our mobile app:.
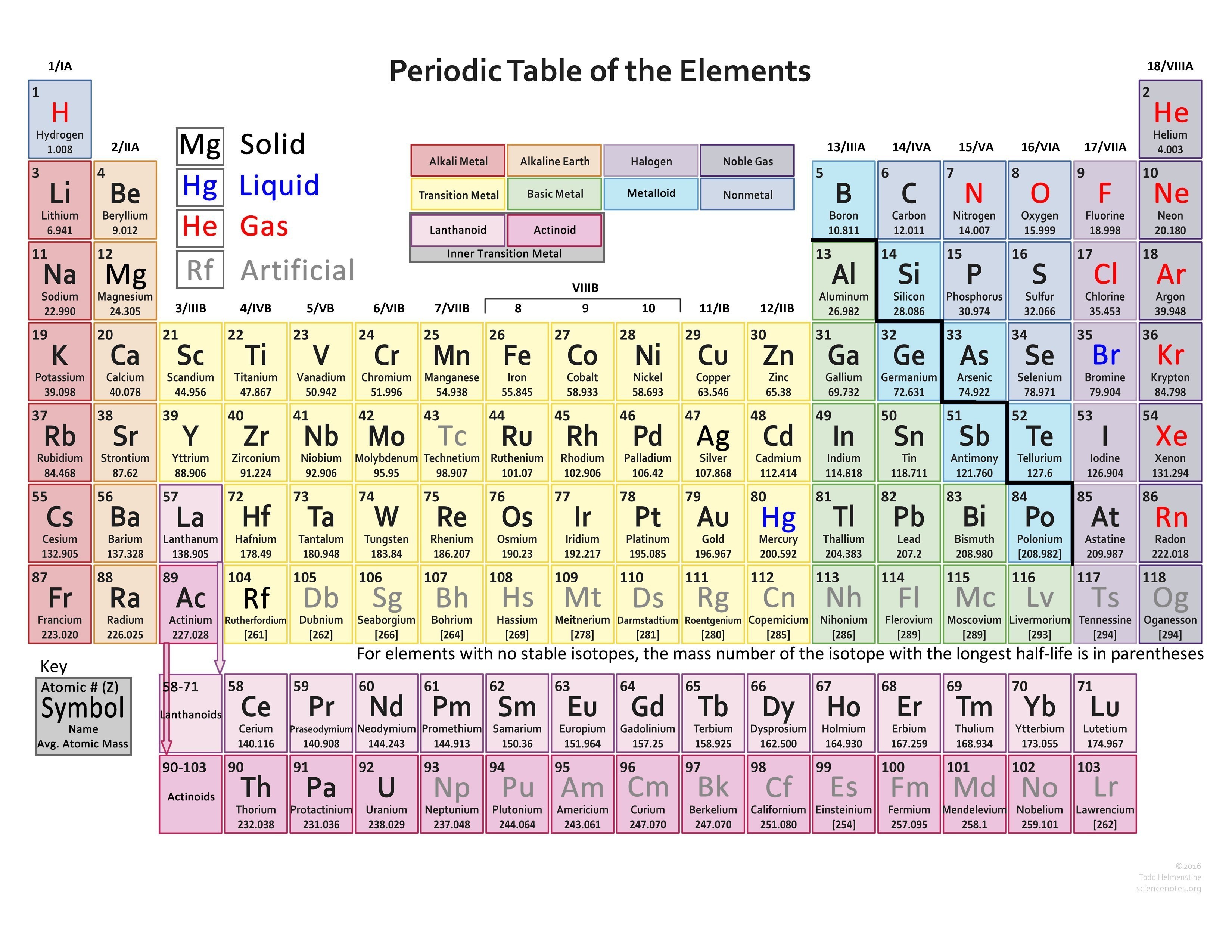
Find Various Types of Valency of Elements Valencies of 118 Elements
The capacity of an atom of an element to form chemical bonds is known as its valency .The valency of an element decides the number of other atoms which can combine with one atom of that element. For example, the valency of carbon is 4 and that of hydrogen is 1. So, one atom of carbon can combine with four atoms of hydrogen to form a methane.

Class 9 Chemistry Chapter 3 Valency Atoms and Molecules YouTube
Textbook Solutions CBSE Notes An Introduction to Valency Chart Valency can be defined as combining the power of an element or radical. The valency chart consists of the list of valencies of the element. We know the chemical formula of salt and water is NaCl and H2O respectively.

ICSE Class 9 Chemistry Additional Charts Reference Valency Chart PDF
This table of element valences includes the maximum valence and most common valence values. Use this is a reference with a periodic table.
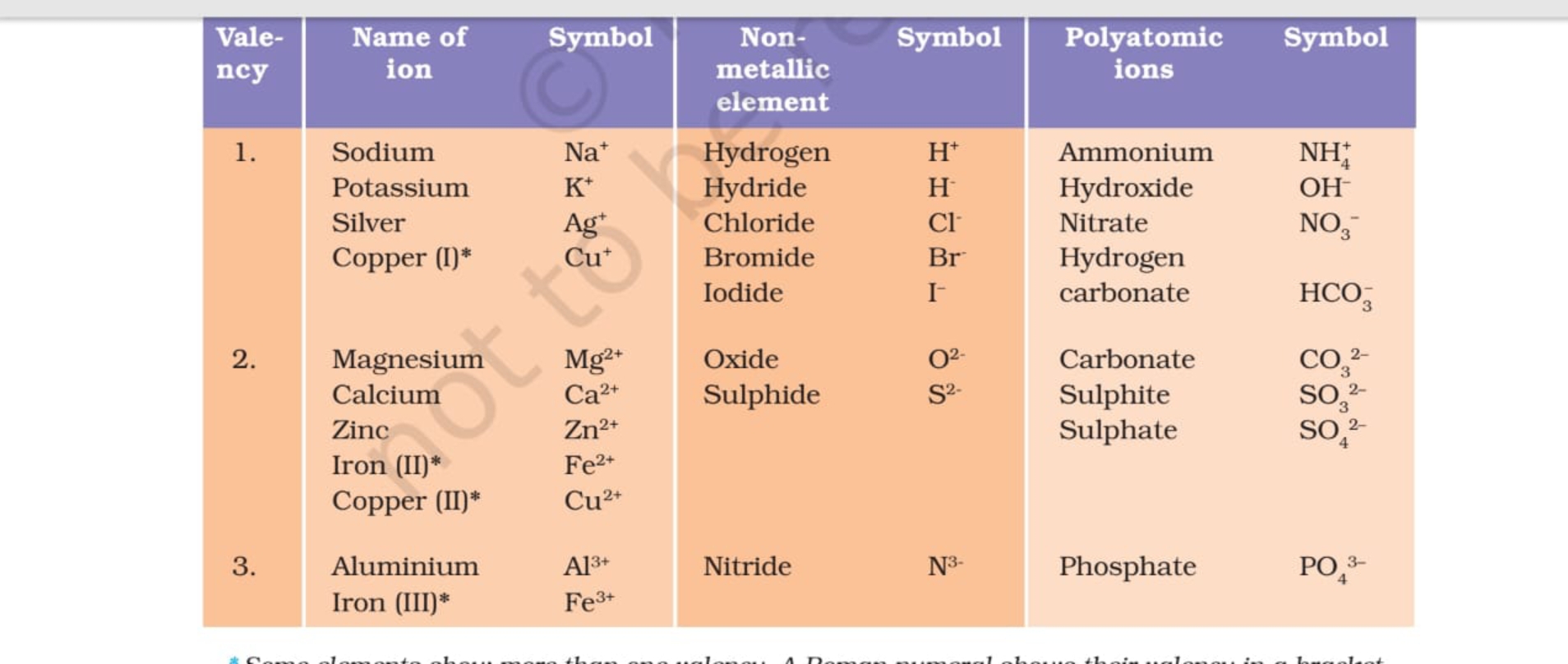
Valency Table Science Notes Teachmint
Next Video : https://youtu.be/dM6zpNKeyCUPrevious Video : https://youtu.be/3Y0Ul89FVaoChapter 3 "Atoms and Molecules" Playlist : https://www.youtube.com/wat.

Valency of an element, structure of the atom, class 9 science, chemistry YouTube
What is Valency? Valency is the measure of the combining capacity of atoms or molecules. Therefore, it is the capacity of an atom of a single element to react and combine with particular numbers of atoms of another element. Browse more Topics under Structure Of Atom Introduction: Structure of Atom Atomic Number Bohr's Model of Atom
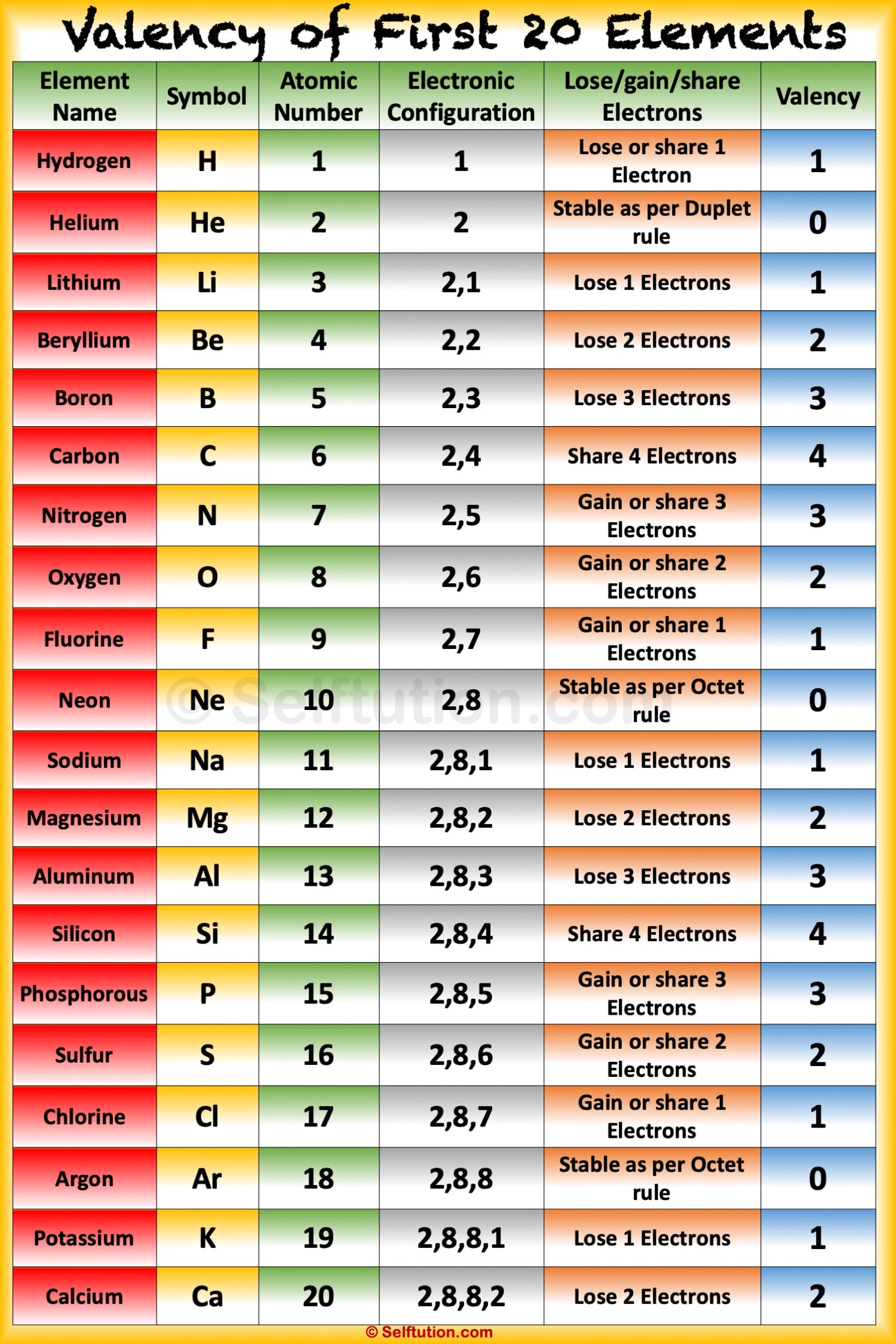
Valency and Variable Valency Valence Shell and Electrons » Selftution
The valence electron of an atom take part in a chemical reaction because they have more energy than all the inner electrons. For Example: (1) Sodium (Z=11) The electronic configuration of sodium is K L M
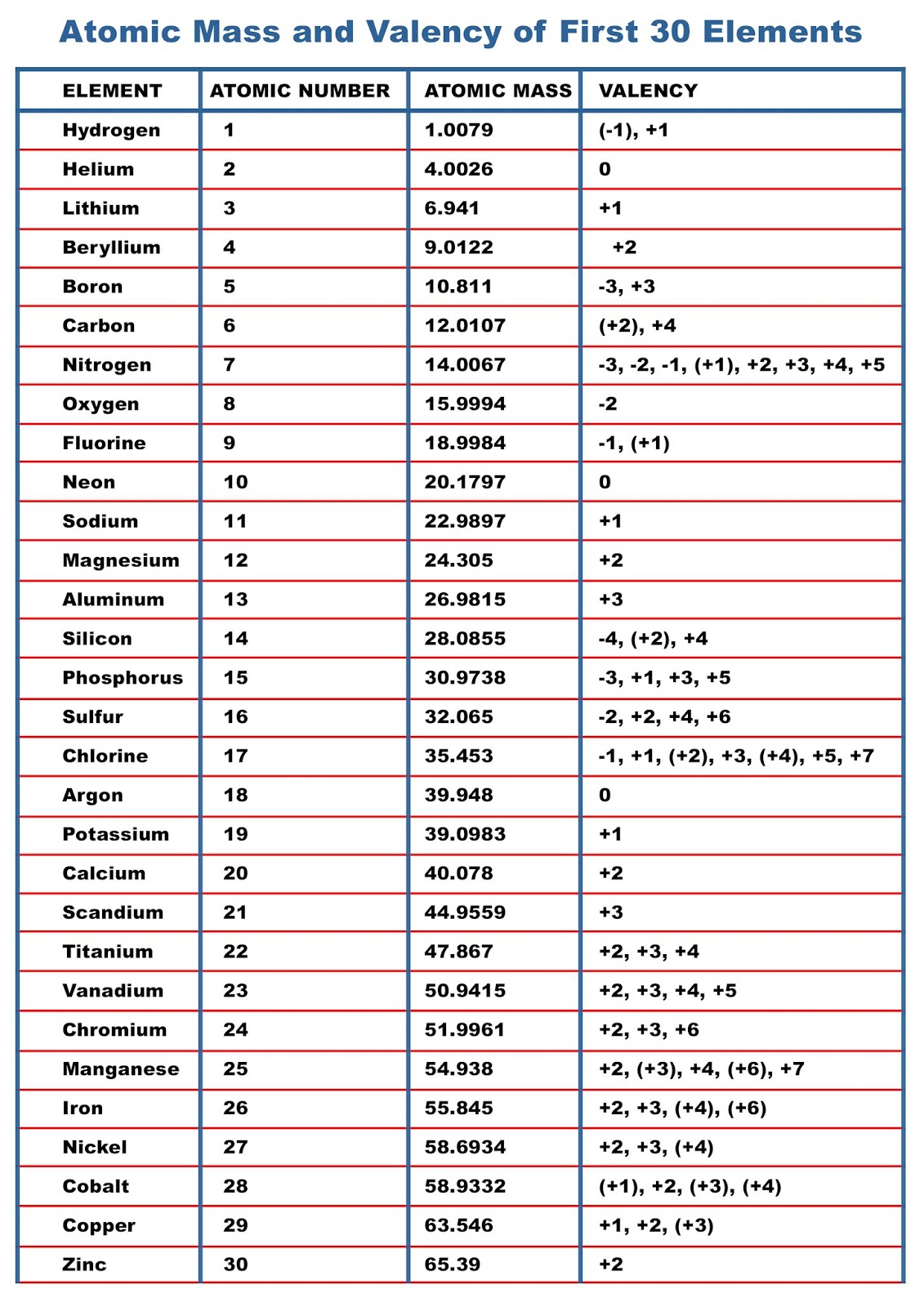
Atomic Mass of Elements Atomic Mass and Valency of First 30 Elements
Valency or Valence of an element is a measure of an atom's ability to combine with other atoms to create molecules or chemical compounds. The characteristics of an element that indicate how many more atoms can join one of its atoms in a covalent bond are known as valence, or valency, in chemistry.

valency table for class 9 Brainly.in
Tr. Vanessa Class Details IX B Science More from Tr. Vanessa (20) Study Material Ans Pg 1 x-a Science 0 Likes 22 Views T Tr. Vanessa Mar 18, 2022 Study Material Pg 1 class-8th Science 0 Likes 41 Views T Tr. Vanessa Feb 22, 2022 Test Test 2 class-7th Geography 0 Likes