Bohr model diagram of Aluminium Al in atomic physics Stock Vector
Bohr Model Of Aluminum PNG Transparent Images, Pictures, Photos PNG Arts
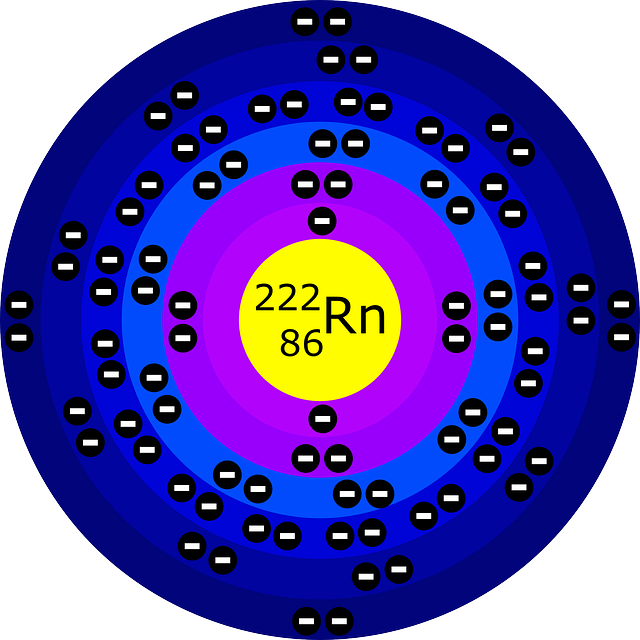
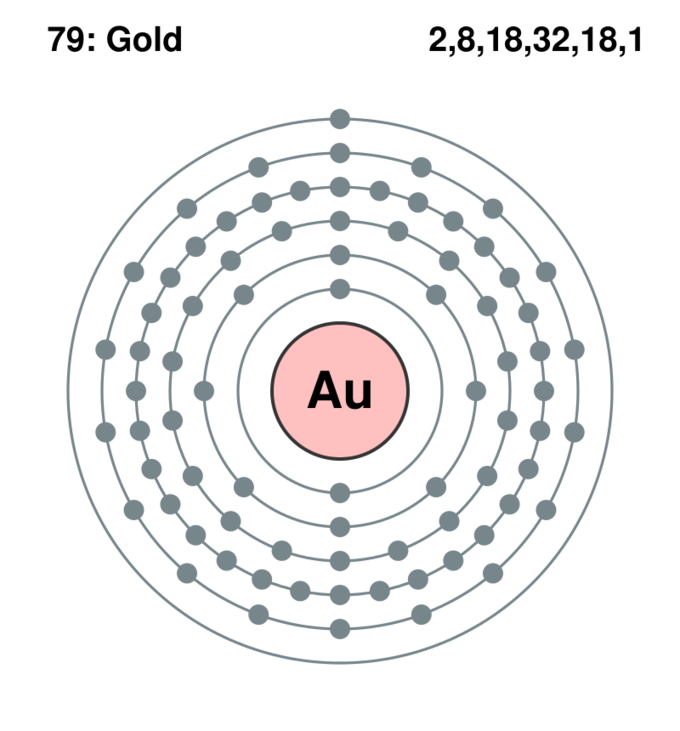
The Bohr model describes the structure of an atom as a central nucleus containing protons and neutrons, with electrons orbiting in specific energy levels around it. Electrons can jump between these energy levels by absorbing or emitting energy. This model helps us understand basic atomic properties and electron behavior in atoms. K Shell
Electron Configuration for Aluminum (Al, Al3+ ion)
9.4: The Bohr Model - Atoms with Orbits is shared under a CK-12 license and was authored, remixed, and/or curated by Marisa Alviar-Agnew & Henry Agnew. LICENSED UNDER. Bohr's model suggests that each atom has a set of unchangeable energy levels, and electrons in the electron cloud of that atom must be in one of those energy levels.

18+ Aluminum Atome
May 27, 2023 by Jay Rana What is a Bohr model? Bohr model is a structural model in which the negatively charged electrons revolve around the positively charged nucleus. This is similar to the planets revolving around the sun, except that the orbits are non-planar. The electrons move in a fixed orbits (shells) and each orbit has a fixed energy.
Aluminum Bohr Model Diagram, Using The Main Group Elements Of The
Category: Science & Tech Key People: Niels Bohr Related Topics: atom See all related content → Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.
3d model of aluminum atom 3d render of atom structure of aluminum
Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that Bohr's model was taken seriously, despite the many assumptions that Bohr needed to derive it. Figure \(\PageIndex{1}\): Quantum numbers and energy levels in a hydrogen atom.
Aluminum Bohr Model ClipArt Best
Aluminum has 2 electrons in its first shell, 8 in its second and 3 in its third.Check me out: http://www.chemistnate.com

Atomic structure of aluminum Brainly.in
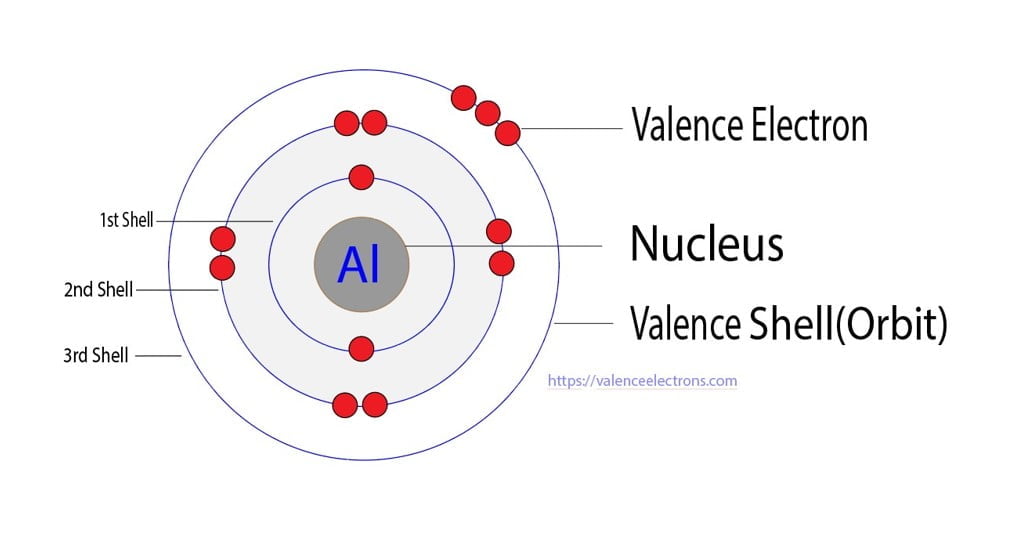
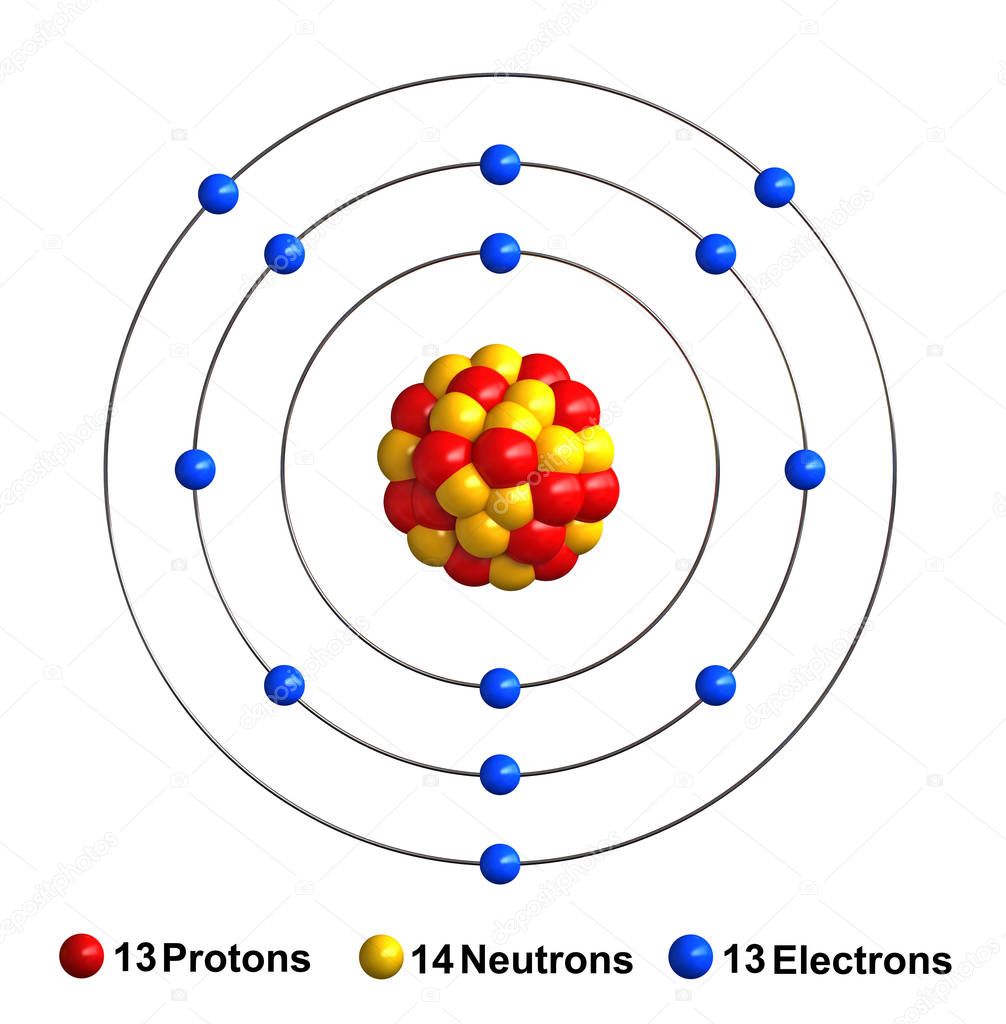
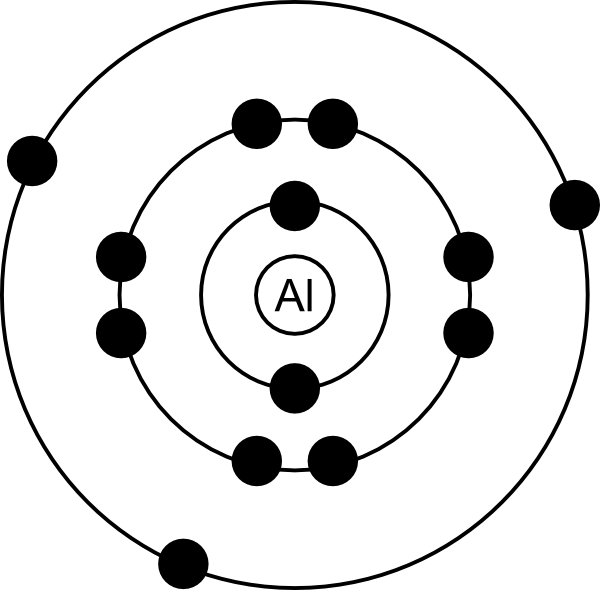
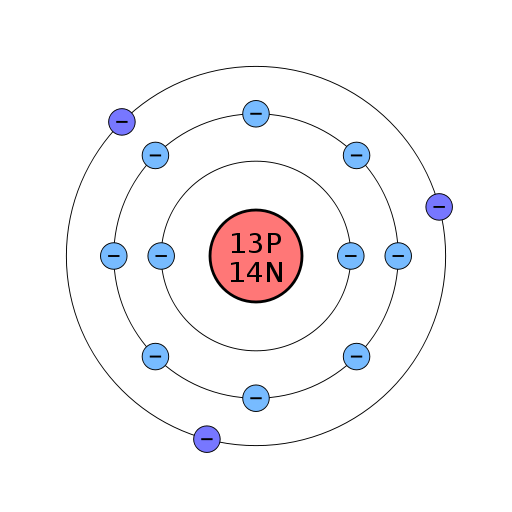
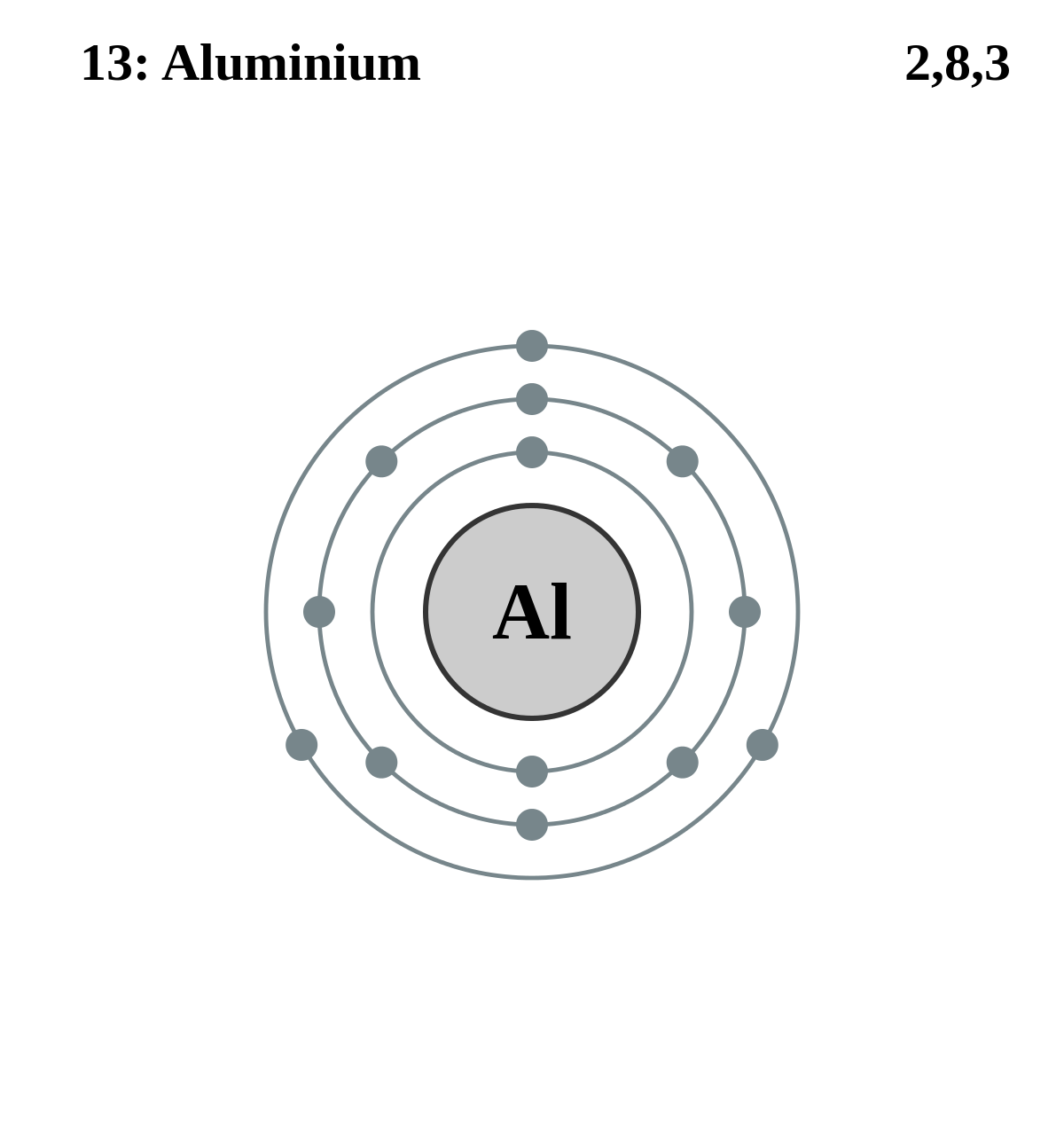
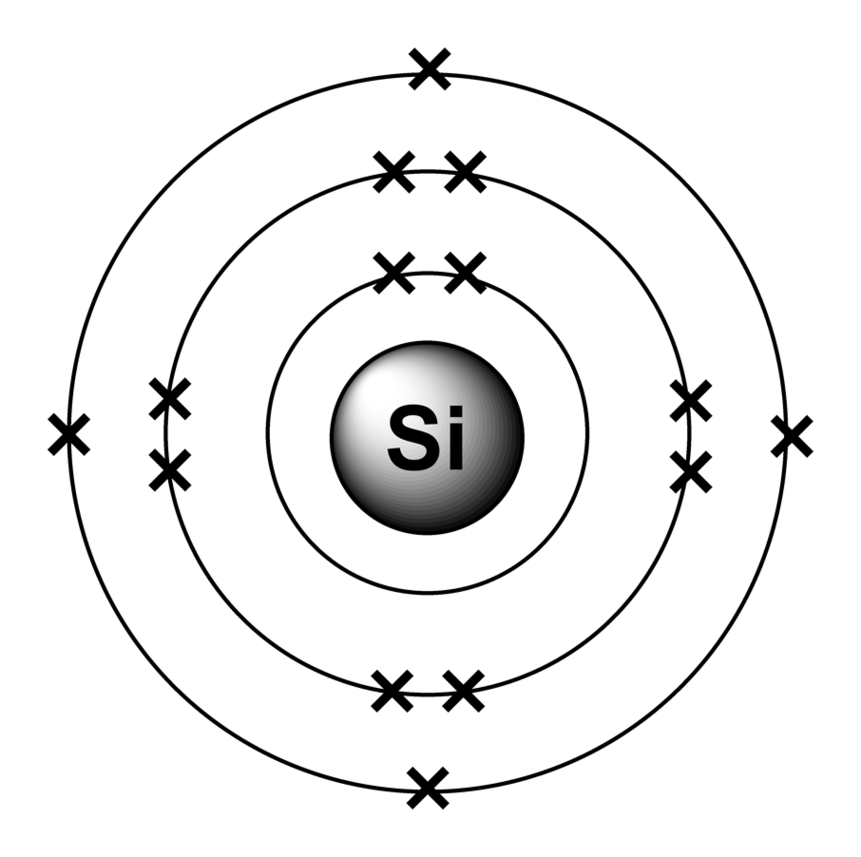
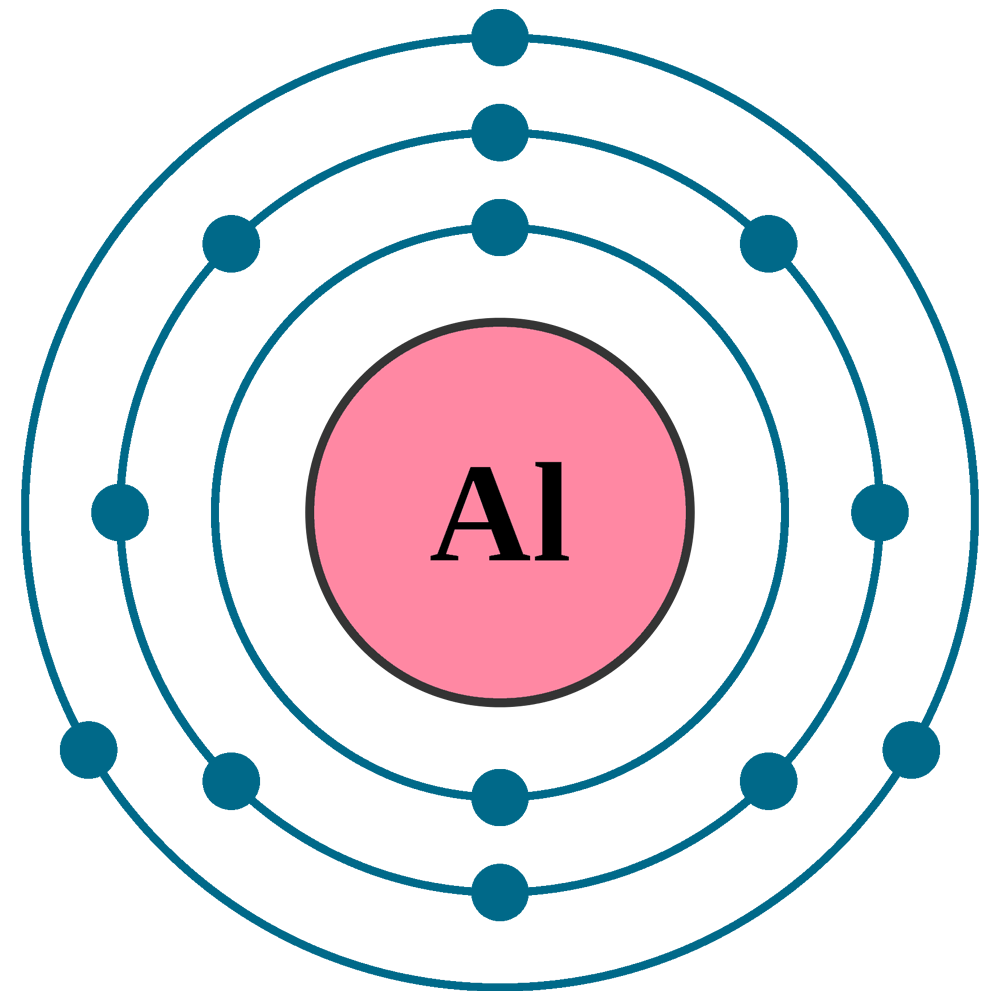
The Bohr model of an aluminum atom consists of 13 protons and 14 neutrons in the nucleus, with 13 electrons orbiting in three energy levels. The first energy level holds 2 electrons, the second holds 8, and the third holds 3.

Bohr Model Aluminium Atom Electron Structure Stock Vector (Royalty Free
What is the Bohr model for aluminum? A Visual Depiction: A Bohr model is a way of visually depicting the structure of an atom of a particular element. An atom is the main component of an.
Bohr Model Of Aluminum PNG Transparent Images, Pictures, Photos PNG Arts
which is identical to the Rydberg equation in which R ∞ = k h c. R ∞ = k h c. When Bohr calculated his theoretical value for the Rydberg constant, R ∞, R ∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that.

Bohr Model Of Aluminum Chemistry Atom Model Bohr Model Atom Model My
Figure 7.3.2 7.3. 2: The emission spectra of sodium and mercury. Sodium and mercury spectra. Many street lights use bulbs that contain sodium or mercury vapor. Due to the very different emission spectra of these elements, they emit light of different colors. The lines in the sodium lamp are broadened by collisions.
:max_bytes(150000):strip_icc()/aluminiumatom-58b602655f9b5860464c6f7d.jpg)
Atom Diagrams Electron Configurations of the Elements
The Bohr diagram, also known as the Bohr model or the planetary model, is a simplified representation of an atom's electron configuration. It was developed by Niels Bohr in 1913 and successfully explained certain properties of atoms, such as the line spectra of hydrogen.
Bohr Model Of Aluminum PNG Transparent Images, Pictures, Photos PNG Arts
The Bohr Model of Aluminum (Al) has a nucleus that contains 14 neutrons and 13 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Aluminum contains 3 electrons that also called valence electrons. Page Contents show How to draw Bohr Model of Aluminum (Al)?
Bohr Model Of Aluminum PNG Transparent Images, Pictures, Photos PNG Arts
Overview of the Bohr Model Niels Bohr proposed the Bohr Model of the Atom in 1915. Because the Bohr Model is a modification of the earlier Rutherford Model, some people call Bohr's Model the Rutherford-Bohr Model. The modern model of the atom is based on quantum mechanics.
Bohr Model of Aluminum Transparent Background PNG PNG Arts
The Bohr model represents the particle nature of electrons. So, it's easy to see that the atom above contains two electrons. As we'll discuss later in the article, atomic electrons exist at specific energy levels. The Bohr model represents these energy levels as rings. We can tell that the two electrons in the model above are at the same energy.
Aluminum Bohr Model ClipArt Best
The Aluminum Bohr Model is a comprehensive atom model that has become a cornerstone of modern chemistry. Developed in 1913 by Danish physicist Niels Bohr, this model is based on the idea that electrons orbit the nucleus of an atom in distinct layers or shells.
Bohr Model of Aluminum PNG Free Download PNG Arts
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table. 2). You will get the detailed information about the periodic table which will convert a newbie into pro. 3). You will also get the HD images of the Periodic table (for FREE).